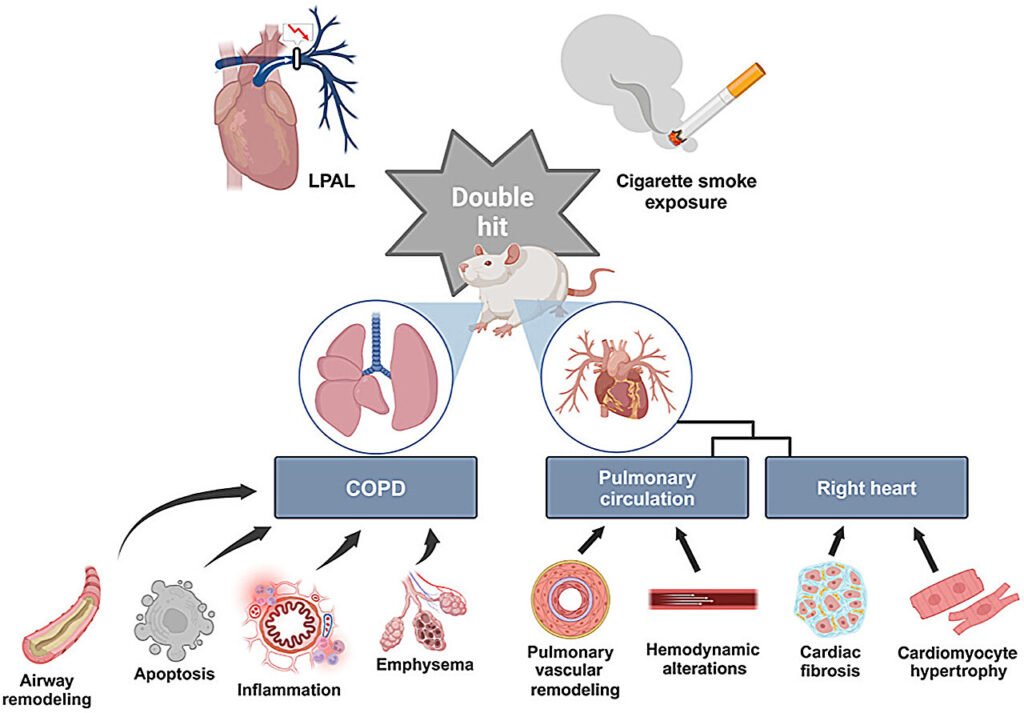
Researchers have recently unveiled a groundbreaking development in the field of chronic obstructive pulmonary disease (COPD) research—a novel rat model that closely mirrors the pathological features and physiological changes associated with COPD-associated cor pulmonale in humans. This innovative model showcases key characteristics such as chronic lung inflammation, pulmonary hypertension, and right ventricular hypertrophy, offering a promising avenue for advancing our understanding of this complex disease.
Published in The American Journal of Pathology, the study highlights the potential of this rat model to unlock the intricate interactions between lung and heart pathology, ultimately leading to improved patient outcomes. COPD is a prevalent chronic respiratory condition characterized by persistent symptoms and airflow limitation, ranking as the third-leading cause of global mortality according to the World Health Organization. In 2019 alone, approximately 3.23 million deaths worldwide were attributed to COPD.
Of particular concern is the development of cor pulmonale in COPD patients, a condition impacting around 6% of individuals with COPD each year. Cor pulmonale, characterized by right ventricular dysfunction stemming from pulmonary disease, significantly worsens the prognosis of COPD and poses a substantial economic burden. However, progress in therapeutic research has been hindered by the absence of accurate animal models mimicking the intricate interplay between COPD and cor pulmonale.
Lead investigator Tao Wang emphasizes the urgent need for a reliable animal model to study COPD-associated cor pulmonale more effectively. By meticulously developing and characterizing this novel rat model, the researchers induced the disease state through chronic cigarette smoke exposure and left pulmonary artery ligation, followed by a thorough analysis of physiological, histological, and molecular aspects. The results demonstrated a faithful replication of COPD-associated features, including pulmonary dysfunction, emphysema, and inflammatory infiltration.
Furthermore, the model successfully reproduced key aspects of cor pulmonale, such as right ventricular hypertrophy, fibrosis, capillary rarefaction, and hemodynamic changes associated with pulmonary hypertension. The researchers identified inflammation and oxidative stress pathways as crucial players in disease progression, suggesting their potential as therapeutic targets. Co-lead investigator Lingdan Chen underscores the significance of this rat model in deepening our understanding of COPD-associated cor pulmonale and advancing therapeutic strategies.
The newfound rat model is poised to unveil novel molecular insights and therapeutic targets, offering hope for more effective treatment options for patients with COPD-associated cor pulmonale. Co-investigators Zhuoji Ma and Suiyang Tong express enthusiasm for the model’s potential to accelerate the discovery of innovative therapeutic approaches in addressing this challenging condition.
In conclusion, the development of this novel rat model represents a significant milestone in COPD research, providing a valuable tool for unraveling the complex mechanisms underlying COPD-associated cor pulmonale. By shedding light on the disease’s pathophysiology and identifying potential therapeutic targets, this model paves the way for transformative advancements in COPD treatment.