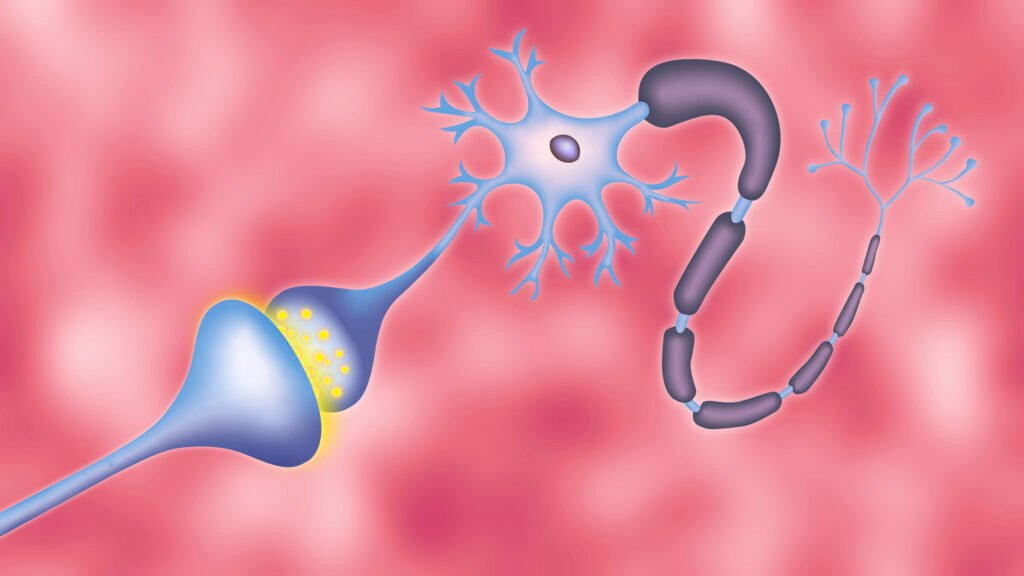
The intricate processes of how we think, feel, remember, and move all involve synaptic transmission, where chemical signals are passed between nerve cells using vesicles. A recent breakthrough in neuroscience research has provided a detailed model of the vesicle cycle, shedding light on the inner workings of the brain.
Published in Science Advances, a collaborative study between researchers at the Okinawa Institute of Science and Technology (OIST) in Japan and the University Medical Center Göttingen (UMG) in Germany utilized a unique computational modeling system. This system considers the complex interactions of vesicles, their cellular environments, activities, and interactions to create a realistic depiction of how vesicles support synaptic transmission.
The model developed by the researchers predicts parameters of synaptic function that were previously difficult to test experimentally, opening up new avenues for neuroscience investigations. Professor Erik De Schutter from OIST emphasized the importance of integrating and interpreting various types of data to understand the complexities of the brain.
Over 20 years of research on synapses has led to the development of this model, which provides a better molecular and spatial understanding of the vesicle cycle. This advancement allows for faster simulations applicable to different cell types and scenarios, making it a significant step towards comprehensive cell and tissue simulations.
The synaptic vesicle cycle involves the release of neurotransmitters at a synapse to transfer information between cells. Vesicles containing neurotransmitters dock at the membrane, fuse to release their contents, and are then recycled. The process is initiated by electrical stimulation in the brain and is driven by a complex signaling cascade.
Research on the vesicle recycling process in hippocampal synapses revealed insights into behavior at high stimulation frequencies. The model showed that the vesicle cycle can operate at frequencies beyond what is typically found in nature, highlighting the roles of key proteins synapsin-1 and tomosyn-1 in regulating vesicle release from the reserve pool.
The efficiency of the vesicle cycle was found to rely on molecular tethering, where vesicles are physically connected to the membrane for rapid docking and neurotransmitter release. These discoveries deepen our understanding of vesicle recycling, a process implicated in various neurological diseases.
As the models are expanded, the potential applications for developing new therapeutics and advancing our understanding of brain function are vast. This research paves the way for future studies on synaptic transmission and neurological disorders.
For more information, you can refer to the study published in Science Advances by Andrew Gallimore et al., titled “Dynamic Regulation of Vesicle Pools in a Detailed Spatial Model of the Complete Synaptic Vesicle Cycle.” (DOI: 10.1126/sciadv.adq6477)
This content was provided by the Okinawa Institute of Science and Technology and can be accessed at their official website for further details.