Researchers have long been interested in the STING pathway as a potential tool to activate the immune system against cancer. The pathway, crucial for the body’s defense against pathogens, can be manipulated to trigger an immune response that targets cancer cells. However, a recent study published in the journal Nature Chemical Biology has shed new light on the complexities of STING inhibition, led by biochemist Lingyin Li.
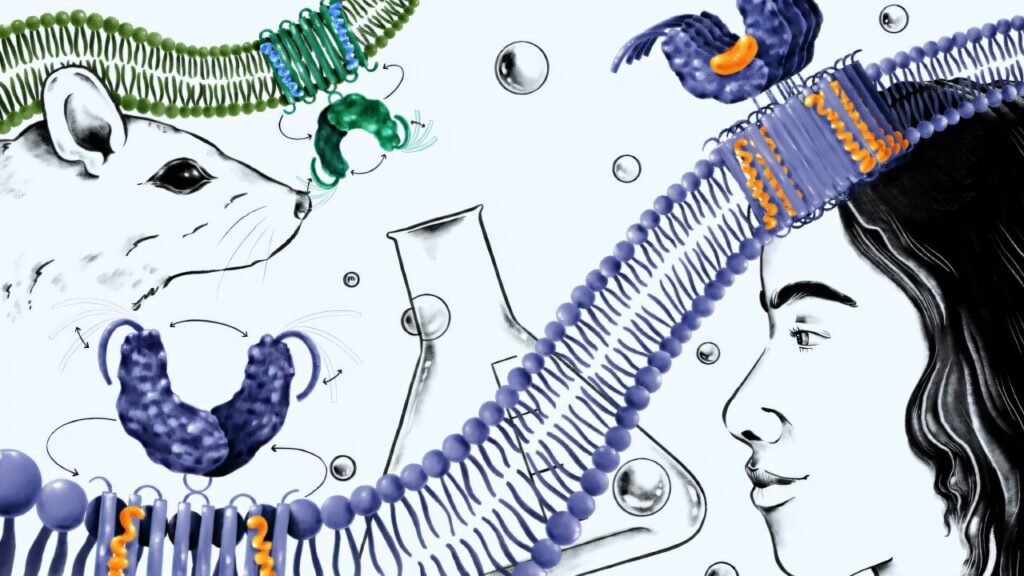
Traditionally, research on STING has focused on activating the pathway to recruit immune cells to attack tumors. However, inhibiting the pathway has been largely overlooked, despite growing evidence suggesting that overactivation of STING could lead to the immune system attacking healthy cells. This dual nature of STING activation and inhibition presents a challenging but promising target for drug development, especially in the context of human biology.
The study evaluated the effectiveness of H-151, a potent inhibitor of human STING that has shown promise in reversing cognitive decline in mice. However, the study found that H-151 failed to block human STING signaling in purified human blood cells. This discrepancy was attributed to a fundamental difference in the target site of H-151 between human and mouse STING, making drug development challenging in humans.
To address this disparity, Li’s team delved into the essential steps of human STING signaling and identified oligomerization as a crucial checkpoint before activation. Inspired by STING’s natural autoinhibitory mechanism, the team developed a molecule that mimics this mechanism, preventing STING from forming the large complexes necessary for immune activation in humans.
The research underscores the importance of developing STING inhibitors tailored specifically for humans. By uncovering a distinct druggable pocket in human STING, the study provides a roadmap for identifying targets that can prevent STING autoimmunity. This precision targeting of STING may open up new treatment avenues beyond cancer immunotherapy, including potential applications in neurodegenerative and autoimmune diseases.
Looking ahead, the Li lab plans to explore the potential of STING inhibition in diverse therapeutic areas and advance the development of human-ready STING inhibitors for future clinical trials. By understanding the intricacies of STING signaling and inhibition in humans, the researchers aim to expand the scope of STING-targeted therapies and pave the way for novel treatment options in various disease settings.
As the research progresses, the findings from this study will likely have far-reaching implications for the field of immunotherapy and drug development. The study not only highlights the importance of species-specific drug targeting but also underscores the need for a nuanced understanding of immune pathways to harness their therapeutic potential effectively.