Scientists at Monash University have made a groundbreaking discovery that could revolutionize the treatment of nerve pain and ischemic diseases. By identifying novel drug candidates targeting the adenosine A1 receptor (A1R), the research team has paved the way for more effective and safer therapies for conditions caused by tissue stress and inflammation.
Neuropathic pain, a type of nerve pain, is often associated with inflammation in the peripheral and central nervous systems. On the other hand, ischemia-reperfusion injury occurs when blood flow is restored to oxygen-deprived tissues, such as after a heart attack. Both conditions can be debilitating and challenging to treat effectively.
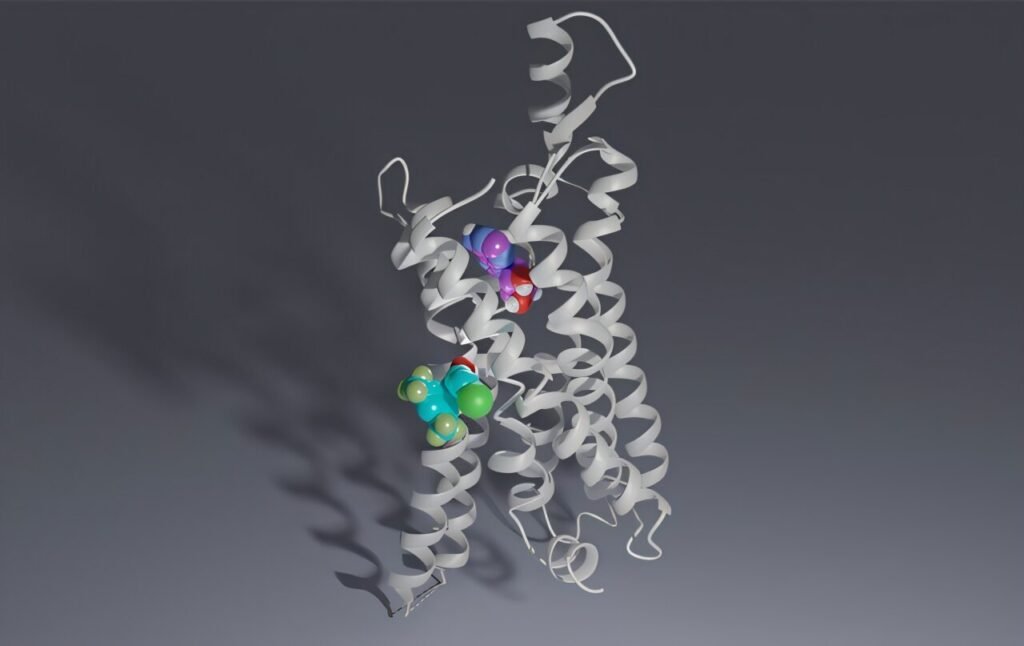
The study, published in PNAS and conducted in collaboration with Sweden’s Uppsala University, focused on developing drug-like candidates that specifically target the A1R subtype. A1Rs are crucial for neuron communication in the brain and heart, making them promising targets for conditions related to tissue stress and inflammation.
The research team utilized advanced technologies to discover a new class of “subtype-selective A1R positive allosteric modulators” (PAMs). Unlike traditional agonists that fully activate A1R and may cause unwanted side effects, these PAMs act as a “dimmer switch,” subtly enhancing the receptor’s response only when necessary. This precise modulation offers more control and fewer side effects, making them promising candidates for drug development.
Dr. Anh Nguyen, one of the study’s co-first authors, highlighted the significance of the discovery in overcoming the challenges associated with targeting A1R. By modulating neuron activity without causing cardiac reactions, the newly identified drug candidates could lead to safer and more effective treatments for a range of conditions.
The success of the study was made possible by advancements in drug screening technologies and computational techniques. Cryo-electron microscopy played a crucial role in identifying the architecture of the A1R allosteric site, while molecular dynamics simulations helped model the membrane environment and screen compound libraries effectively.
Moving forward, the research team plans to further develop the lead compounds and test their efficacy in preclinical models of neuropathic pain and ischemia-reperfusion injury. The ultimate goal is to advance these findings into clinical trials and develop targeted therapeutics for conditions involving A1R signaling.
This groundbreaking research opens new possibilities for treating nerve pain and ischemic diseases, offering hope for safer and more effective therapies in the future. With continued advancements in drug discovery and technology, the potential for life-changing treatments is within reach.