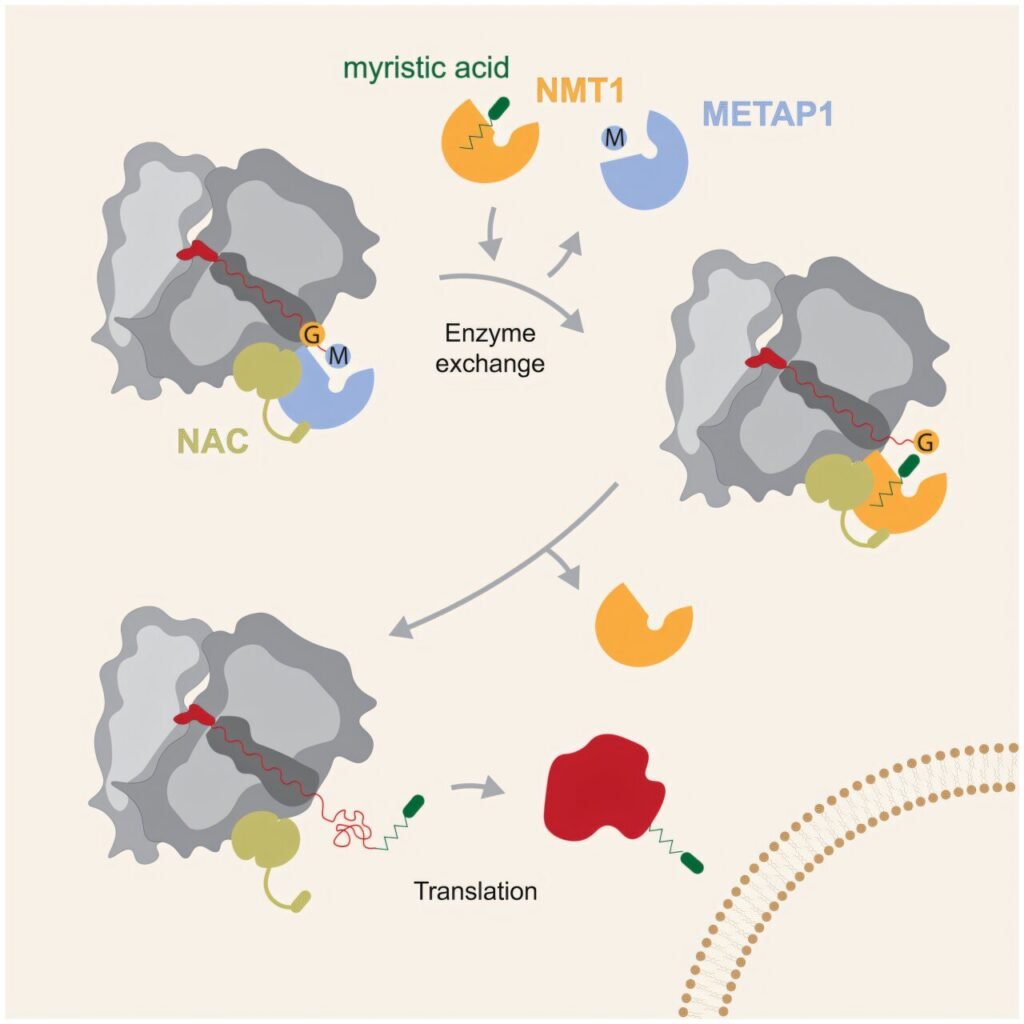
A groundbreaking study led by a team of researchers from the University of Konstanz has uncovered a molecular mechanism that regulates the activity of N-myristoyltransferases (NMTs). These enzymes play a crucial role in biological signaling pathways, and any dysregulation can lead to serious illnesses such as cancer and viral infections.
Proteins are essential components of life, with their production and regulation being key to maintaining the body’s overall health. Understanding how proteins are produced, modified, and interact can provide valuable insights into preventing diseases or developing effective treatments.
In their recent publication in Molecular Cell, researchers from the University of Konstanz, in collaboration with colleagues from ETH Zurich and the California Institute of Technology, delved into the role of NMTs in protein modification. These enzymes chemically modify proteins during their production, and their activity is vital for proper biological functioning. The study not only unveiled the intricate molecular mechanism controlling NMT activity at the cellular level but also identified a promising target for developing improved drugs to combat certain types of cancer and viral infections.
The study revealed that NMTs play a crucial role in protein production by adding myristic acid to nascent proteins, a modification essential for their biological activity. The researchers discovered that a protein complex known as the nascent polypeptide-associated complex (NAC) orchestrates the activity of NMTs on the ribosome, the cellular structure where proteins are produced. The NAC acts as a “grabbing arm,” positioning the enzymes at the ribosomal tunnel where proteins emerge, ensuring the timely addition of myristic acid to the nascent proteins.
Furthermore, the study uncovered a signal motif that controls the exchange of enzymes on the ribosome, allowing for the sequential addition of modifications to nascent proteins. This precise coordination ensures that NMTs can fulfill their function without interference from other enzymes, ultimately paving the way for the development of targeted therapies for diseases involving dysregulated NMT activity.
The research team’s findings offer a new perspective on how NMTs operate within the cell and suggest novel strategies for designing drugs that selectively target these enzymes. By focusing on the interaction between NMTs and the NAC complex, researchers aim to develop more effective treatments for cancer and viral infections, minimizing potential side effects associated with current drug therapies.
This study underscores the importance of understanding the molecular mechanisms governing protein modification and highlights the potential of targeting NMTs for therapeutic interventions. By unraveling the intricacies of NMT regulation, researchers are paving the way for innovative treatments that could revolutionize the management of various diseases in the future.