A groundbreaking study led by Professor Takuya Yamamoto and Professor Yasuhiro Yamada at the University of Tokyo has unveiled a new in vivo system that sheds light on how senescent cells function within living tissues and impact the aging process through complex mechanisms. Published in the prestigious journal Nature Aging, this research provides valuable insights into the role of cellular senescence in aging-related processes.
Cellular senescence, a state of irreversible cell cycle arrest triggered by various stressors, has long been recognized for its dual role as a protective mechanism against cancer and a contributor to age-related diseases. However, the precise physiological significance of senescence in vivo has remained elusive due to the lack of suitable models and the challenges associated with identifying senescent cells in complex tissue environments.
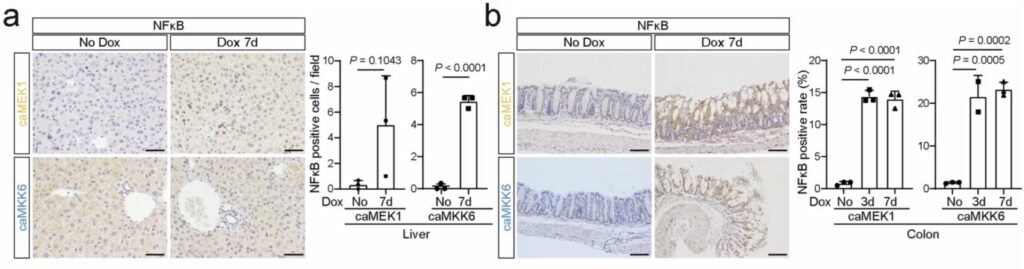
To address this gap, the research team developed innovative mouse models capable of inducing the expression of two key proteins—constitutively active MEK1 (caMEK1) and MKK6 (caMKK6)—known to initiate senescence in vitro. By utilizing a dual-color labeling system that distinguishes primary senescent cells from surrounding secondary senescent cells, the researchers were able to track the behavior of senescent cells at the single-cell level and examine their impact on tissue function.
Both caMEK1 and caMKK6 were found to induce classic hallmarks of senescence in liver and colon tissues, including increased expression of p21, DNA damage responses, and the secretion of senescence-associated factors. Importantly, transcriptomic analyses revealed that senescent cells exhibit diverse gene expression profiles that vary based on tissue type, senescence inducer, and spatial location within the tissue.
One of the most striking findings of the study was the disruption of liver zonation—a critical spatial organization that supports liver function—in caMEK1-induced models. This disturbance led to impaired gene expression related to metabolism, mirroring changes observed in naturally aged tissues. Comparative analyses further confirmed that the gene expression profiles of senescent cells in these models closely resembled those found in aged organisms, underscoring the causal role of senescence in age-related tissue dysfunction.
By elucidating the intricate interplay between primary and secondary senescent cells in vivo, this study provides a comprehensive understanding of how senescent cells operate within living tissues and influence neighboring cells. The development of caMEK1 and caMKK6 mouse models represents a significant advancement in aging research, offering valuable tools for investigating the role of senescence in organismal aging and developing targeted therapeutic interventions.
In conclusion, the research conducted by Professor Yamamoto and Professor Yamada’s team opens up new avenues for exploring the impact of senescent cells on the aging process. By unraveling the complex mechanisms underlying cellular senescence in vivo, this study sets the stage for future investigations and therapeutic strategies aimed at modulating senescence to promote healthy aging.