A groundbreaking study conducted by a team of scientists at UT Southwestern Medical Center has shed light on a crucial stage of neurodevelopment where differentiating neural cells produce fewer ribosomes, impacting protein production. This discovery, published in Nature Cell Biology, provides insight into how mutations affecting ribosome production can lead to neurodevelopmental disorders.
Lead by Michael Buszczak, Ph.D., a Professor of Molecular Biology at UT Southwestern, the research team identified a significant decrease in ribosome levels during neuroepithelial differentiation, an early step in human brain development. This decrease makes differentiating cells more susceptible to changes in ribosome biogenesis, highlighting a vulnerable period in brain cell development.
Collaborating with researchers from UT Southwestern and international partners, the study focused on a group of neurodevelopmental disorders characterized by intellectual disability, muscle tone issues, hearing and vision impairments, and small brain size. These disorders are linked to mutations in the AIRIM gene, which plays a crucial role in ribosome generation. However, the mechanism through which these mutations cause these features has remained unknown.
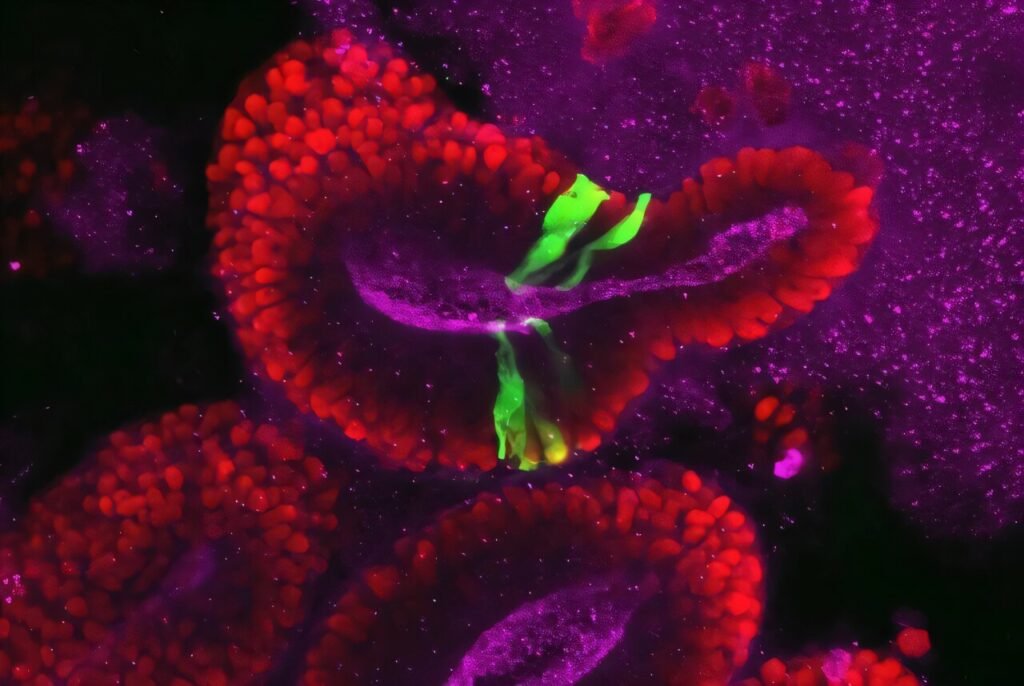
Using cutting-edge techniques to create brain organoids that mimic the development of organs, the researchers genetically manipulated cells with defects in the AIRIM gene to form brain organoids. Comparing these organoids to defect-free cells revealed that mutated organoids were smaller and experienced more cell death by day 15 of development, a critical stage in cell differentiation.
Further analysis showed that both types of organoids exhibited reduced ribosome production, with mutated organoids showing even lower levels. This deficiency in ribosomes led to decreased production of specific proteins involved in cell survival and differentiation. By stimulating cells to increase mTOR protein activity, which promotes protein production, the researchers were able to “rescue” cells with genetic defects, restoring normal organoid size and protein production levels.
Dr. Buszczak suggested that similar interventions could potentially treat neurodevelopmental disorders caused by ribosome deficiencies in patients before birth, potentially preventing the onset of symptoms associated with these conditions. Future research will explore whether other neurodevelopmental disorders linked to genetic mutations in ribosome-related genes are also connected to natural dips in ribosome production during development.
This groundbreaking study opens up new avenues for understanding and potentially treating neurodevelopmental disorders by targeting ribosome deficiencies during critical stages of brain development. The findings provide hope for future interventions that could improve outcomes for individuals affected by these conditions.