Researchers at Children’s Hospital of Philadelphia (CHOP) have introduced a groundbreaking antibody-drug conjugate (ADC) known as CDX0239-PBD, which has shown remarkable efficacy against cancers that express the anaplastic lymphoma kinase (ALK) protein on the cancer cell surface. This innovative therapy has demonstrated complete and lasting tumor responses in preclinical models of neuroblastoma, rhabdomyosarcoma, and colorectal carcinoma, as reported in a recent study published in Nature Communications.
The development of CDX0239-PBD represents a significant advancement in the field of precision medicine, offering new hope for both childhood and adult cancer patients. By targeting the ALK protein, which is often mutated in various cancer types, this therapy has the potential to improve patient outcomes and reduce the harmful side effects associated with traditional treatments.
Dr. Yael P. Mossé, a renowned expert in pediatric oncology and leader of the Neuroblastoma Developmental Therapeutics Program at CHOP, played a crucial role in the discovery of ALK gene mutations in neuroblastoma. These findings paved the way for the development of CDX0239-PBD, spearheaded by Dr. Alberto D. Guerra, a fellow in the Division of Oncology at CHOP.
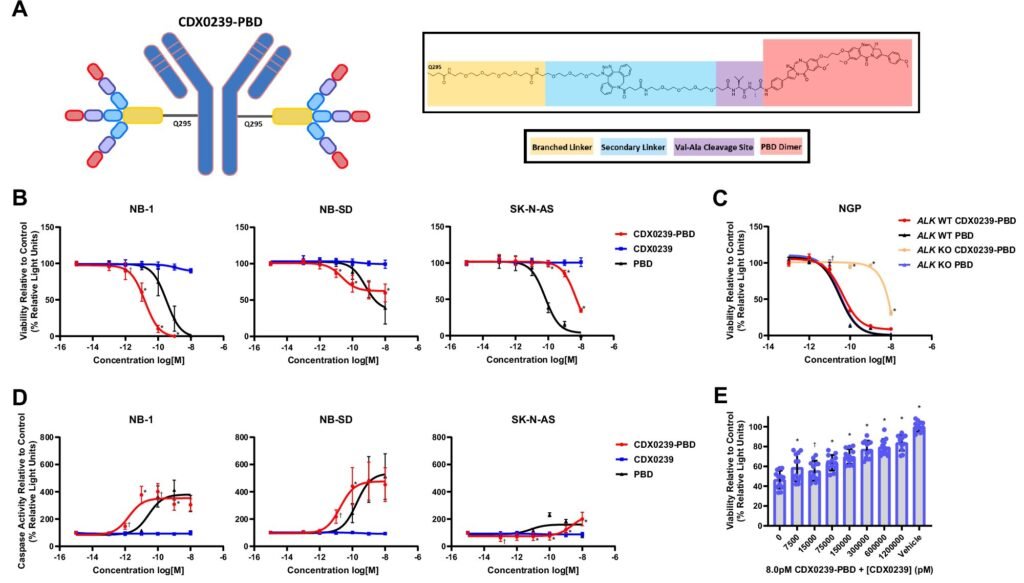
In preclinical studies, CDX0239-PBD combines a humanized antibody targeting ALK with a potent chemotherapy agent called pyrrolobenzodiazepine (PBD) dimer. This unique approach delivers the chemotherapy directly to cancer cells while sparing healthy cells that do not express ALK, resulting in a highly selective and effective treatment strategy. The therapy has shown promising results in targeting a broad range of ALK surface expression levels, making it suitable for a diverse patient population.
Notably, CDX0239-PBD has demonstrated success in eliminating tumors and improving survival rates in drug-resistant models of neuroblastoma, rhabdomyosarcoma, and colorectal carcinoma. Even in cases where conventional treatments have failed, this therapy has shown significant efficacy, highlighting its potential as a versatile and potent treatment option for aggressive cancers.
Further molecular analyses have confirmed the mechanism of action of CDX0239-PBD, showing that it induces DNA damage and activates cell-death pathways within tumors. This targeted approach not only kills cancer cells expressing ALK but also affects neighboring tumor cells through a phenomenon known as the “bystander effect.”
Looking ahead, the research team is focused on refining the technology to meet regulatory standards for clinical trials. Plans are underway for early phase clinical testing of CDX0239-PBD in human patients within the next two years. Additionally, efforts are being made to explore alternative antibodies that can enhance the therapy’s penetration into solid tumor microenvironments.
In conclusion, the development of CDX0239-PBD represents a significant milestone in the field of precision medicine, offering a tailored and effective treatment option for patients with ALK-expressing cancers. This groundbreaking therapy has the potential to revolutionize cancer treatment by improving survival rates and minimizing the side effects of traditional therapies, ultimately enhancing the quality of life for cancer patients post-treatment.