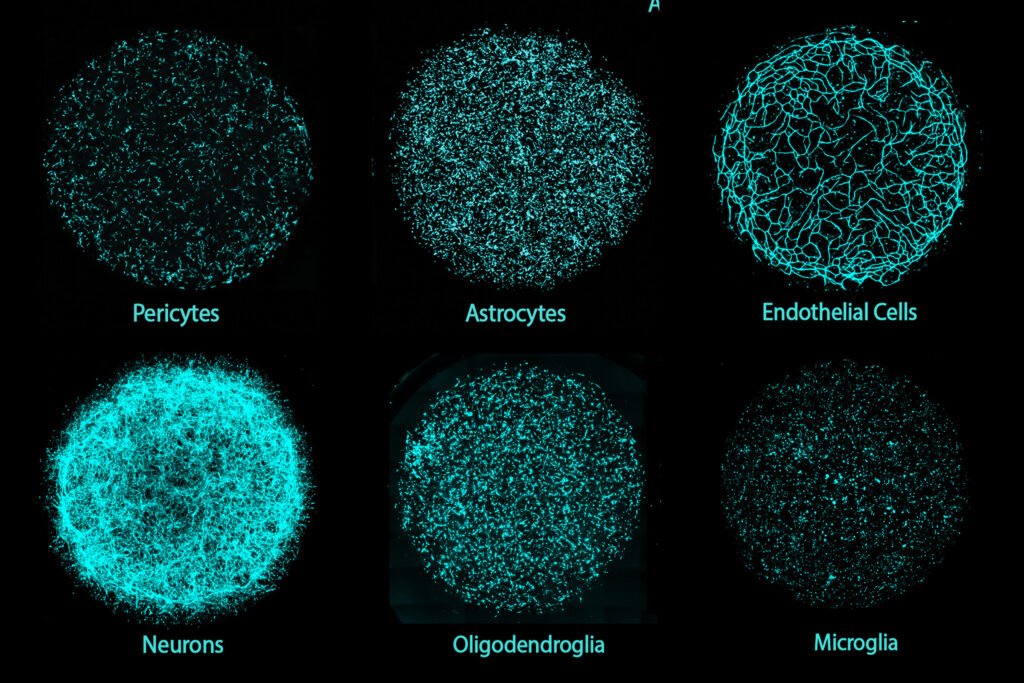
A groundbreaking development in brain tissue engineering has emerged from the laboratories of MIT researchers. The team has successfully created a 3D human brain tissue platform called Multicellular Integrated Brains (miBrains), which integrates all major brain cell types, including neurons, glial cells, and the vasculature, into a single culture. These miBrains are grown from induced pluripotent stem cells obtained from individual donors, making them customizable through gene editing and scalable for large-scale research endeavors.
Despite their diminutive size, smaller than a dime, miBrains hold immense value for researchers and drug developers seeking more sophisticated models to deepen their understanding of brain biology and advance disease treatments.
“The miBrain is the only in vitro system that contains all six major cell types that are present in the human brain,” stated Li-Huei Tsai, Picower Professor and senior author of the study published in the Proceedings of the National Academy of Sciences. The miBrains have already proven their utility by uncovering insights into how a common genetic marker for Alzheimer’s disease influences cellular interactions to drive pathology.
The design of miBrains combines the advantages of simple cell cultures and complex animal models, offering a balance between accessibility and biological relevance. By recreating the intricate interactions between different brain cell types in a 3D tissue environment, miBrains provide a more accurate representation of human brain biology. Moreover, being personalized to individual genomes, miBrains offer a tailored approach to studying specific health conditions and diseases.
The development of miBrains was not without challenges, particularly in creating a suitable substrate to support cell viability and functionality. Drawing inspiration from the brain’s extracellular matrix, the researchers devised a hydrogel-based “neuromatrix” that mimics the brain’s natural environment. This neuromatrix facilitates the assembly of all major brain cell types while promoting the development of functional neurons.
One of the key breakthroughs enabled by miBrains was the study of the APOE4 gene variant, a prominent genetic risk factor for Alzheimer’s disease. By integrating astrocytes carrying the APOE4 variant with other brain cell types, researchers could isolate the contributions of these cells to disease pathology within the complex multicellular environment of miBrains.
Looking ahead, the research team plans to enhance miBrains with additional features to better mimic the complexities of functioning brains, such as incorporating microfluidics for blood vessel flow and single-cell RNA sequencing for improved neuron profiling. The potential applications of miBrains extend to advancing research discoveries and treatment strategies for Alzheimer’s disease and other neurological conditions, with the prospect of personalized medicine on the horizon.
The development of miBrains represents a significant advancement in brain tissue engineering, offering a versatile and highly customizable platform for exploring human brain biology and disease mechanisms. With its modular design and precise control over cellular inputs, miBrains hold promise for accelerating drug discovery, disease modeling, and therapeutic development in the field of neuroscience.