Clinical research is undergoing a digital transformation as the world becomes more interconnected. One significant change in this field is the emergence of decentralized clinical trials (DCTs). Unlike traditional trials that require participants to physically visit hospitals or research centers, DCTs enable individuals to take part in research studies from the comfort of their own homes. This shift in the research paradigm offers numerous advantages, such as increasing patient access to clinical trials and reducing the burden on participants by eliminating the need for travel and in-person visits.
The adoption of decentralized trials has enabled researchers to reach a broader pool of participants and gather data more efficiently. However, this innovative approach is not without its challenges and risks, which are being addressed through the development of new data collection tools. Recent articles published in the Journal of Clinical and Translational Science by researchers from the Medical University of South Carolina (MUSC) shed light on these tools and their impact on improving the integrity and reliability of decentralized trials.
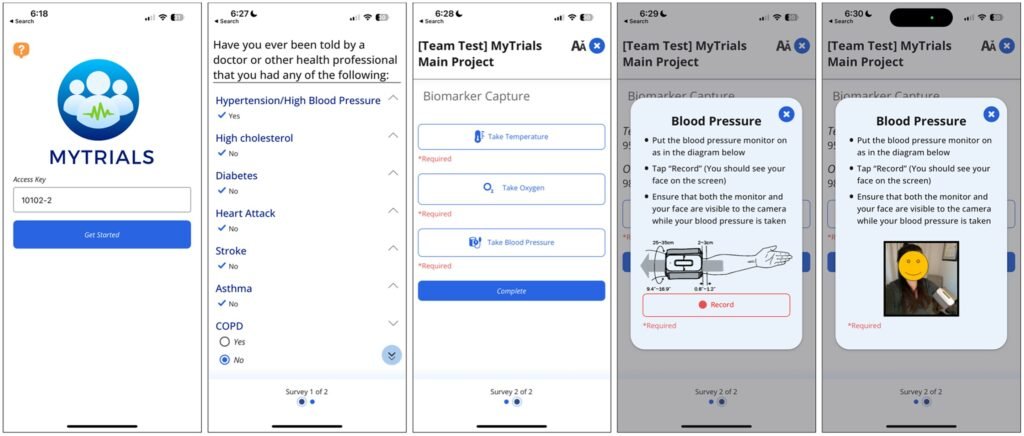
What exactly are decentralized clinical trials? According to Jennifer Dahne, Ph.D., a professor of Psychiatry and Behavioral Sciences at MUSC, decentralized trials bring the research directly to the participants. This approach helps to overcome barriers to participation in clinical research by making it more convenient for individuals to engage in studies from their own homes. Dahne, along with Gaylen Fronk, Ph.D., a postdoctoral fellow in her lab, has been instrumental in developing MyTrials, a system that allows participants to collect and submit health data remotely, thereby enhancing the accessibility and efficiency of DCTs.
The benefits of decentralized trials are manifold. They facilitate faster recruitment processes, broaden the reach of research studies, and enable participants to engage without the constraints of physical travel to clinics. This model also enables researchers to access a more diverse pool of participants, including those from rural areas or underserved communities, leading to more robust data collection and the development of more effective treatments.
Despite the advantages of DCTs, they face challenges related to fraud and bias. The remote nature of these trials makes it difficult to verify the identity of participants, rendering them susceptible to fraudulent activities. In a study conducted by Dahne’s team, a significant portion of survey submissions were found to be fraudulent, highlighting the importance of ensuring data integrity in decentralized trials to avoid biased outcomes and inaccurate findings that could impact medical decisions and public health.
To address these challenges, Dahne and her team developed CheatBlocker, a tool designed to detect fraud early in the trial process by identifying duplicate screening submissions. This tool works seamlessly with REDCap, a widely used software application for data collection, to enhance the security and reliability of decentralized trials. Additionally, Fronk’s research introduces QuotaConfig, a tool that monitors key characteristics during screening to ensure the representativeness of the sample enrolled in the trial, thereby mitigating sampling bias and enhancing the validity of study results.
Moreover, the MyTrials system simplifies the process of data collection by allowing participants to submit health data, such as biomarkers, from home using a smartphone app. This streamlined approach eliminates the need for multiple data collection tools and ensures that information is securely transmitted to researchers via REDCap. The inclusion of a video capture function in MyTrials further enhances data integrity by confirming participant identities and reducing the risk of fraudulent activity.
Looking ahead, Dahne and Fronk envision wider adoption of their tools in decentralized trials to streamline data collection and improve the scientific rigor of research studies globally. Future enhancements to MyTrials may include the collection of additional biomarkers, such as saliva and breath samples, to further expand the capabilities of the platform. By making these tools accessible to researchers worldwide, Dahne aims to empower scientists to conduct more feasible and rigorous scientific investigations that benefit from the advancements in decentralized clinical trials.
In conclusion, the evolution of decentralized clinical trials represents a significant shift in the landscape of clinical research, offering new opportunities for researchers to engage with participants remotely and collect data more efficiently. Through the development of innovative tools like CheatBlocker, QuotaConfig, and MyTrials, researchers are addressing the challenges of fraud and bias in decentralized trials while enhancing data integrity and sample representativeness. As these tools continue to evolve and gain broader adoption, the future of clinical research looks promising, with decentralized trials paving the way for more inclusive, efficient, and reliable scientific investigations.