Spinal cord injury (SCI) is a debilitating condition that can have a profound impact on one’s quality of life, often leading to permanent disability. The limited regenerative capacity of the central nervous system (CNS) poses a significant challenge in treating SCI, as the damaged spinal cord becomes increasingly difficult to repair.
During spinal cord development, neural stem cells differentiate into various neural cells that form complex circuits. However, as the spinal cord matures, these progenitor cells lose their regenerative potential, making adult spinal cord tissue less capable of recovery after injury. Identifying endogenous stem cells with diverse lineage potentials in the adult spinal cord is crucial for advancing repair strategies for SCI.
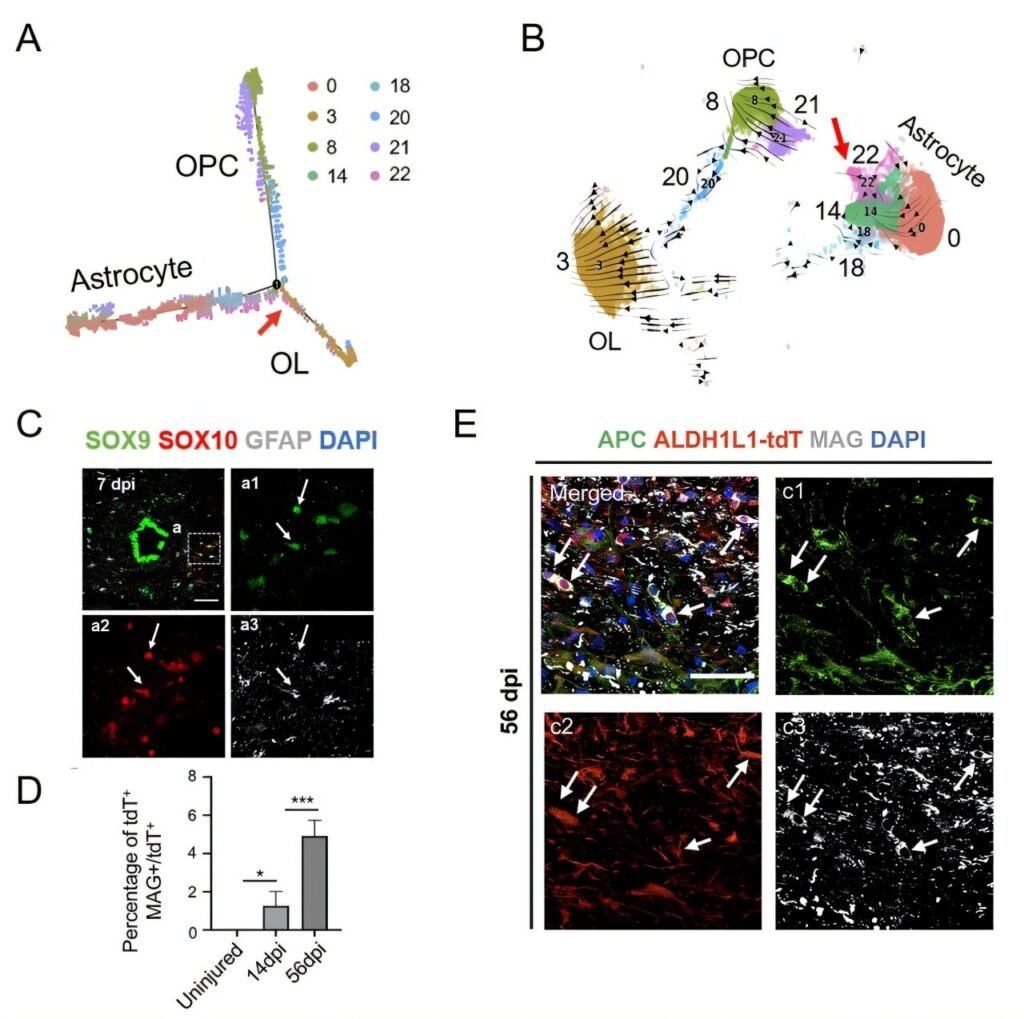
In a recent study published in the Proceedings of the National Academy of Sciences, researchers from the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences investigated the regenerative mechanisms behind SCI repair. By constructing single-cell transcriptomic databases for human spinal cord development and rhesus monkey SCI models, the researchers gained new insights into the behaviors of ependymal and astrocyte cells following SCI.
The study revealed that ependymal cells in the injured spinal cord displayed minimal activation and showed no significant proliferation or cross-lineage differentiation. This lack of reactivity post-SCI was more pronounced in primates than in rodents, suggesting a more restricted regenerative potential in primates.
On the other hand, astrocytes in the injured spinal cord exhibited significant activation and were found to transdifferentiate into oligodendrocytes under injury-induced conditions, contributing to the remyelination process. Key transcription factors such as SOX10 were identified as promoting the conversion of astrocytes into oligodendrocyte lineage cells.
To enhance the regenerative process, the researchers introduced functional material transplantation into the injury microenvironment. This intervention significantly increased the efficiency of astrocyte transdifferentiation into oligodendrocytes, demonstrating the potential of material transplantation in promoting remyelination and repair.
This study sheds light on the limited regenerative capacity of ependymal cells in adult primate spinal cord injury and highlights the transdifferentiation potential of astrocytes. The findings underscore the importance of microenvironmental modulation in enhancing the efficiency of astrocyte-mediated repair, offering promising avenues for future SCI therapies.
In conclusion, the research provides valuable insights into the regenerative mechanisms behind SCI repair and offers a potential strategy for improving outcomes in patients with spinal cord injuries. Further studies in this area will be essential for developing effective therapies to address the challenges posed by SCI.