Low-density lipoproteins (LDL), commonly known as bad cholesterol, have long been a focus of scientific research due to their role in heart disease. However, the intricate inner workings of these microscopic particles have remained elusive—until now.
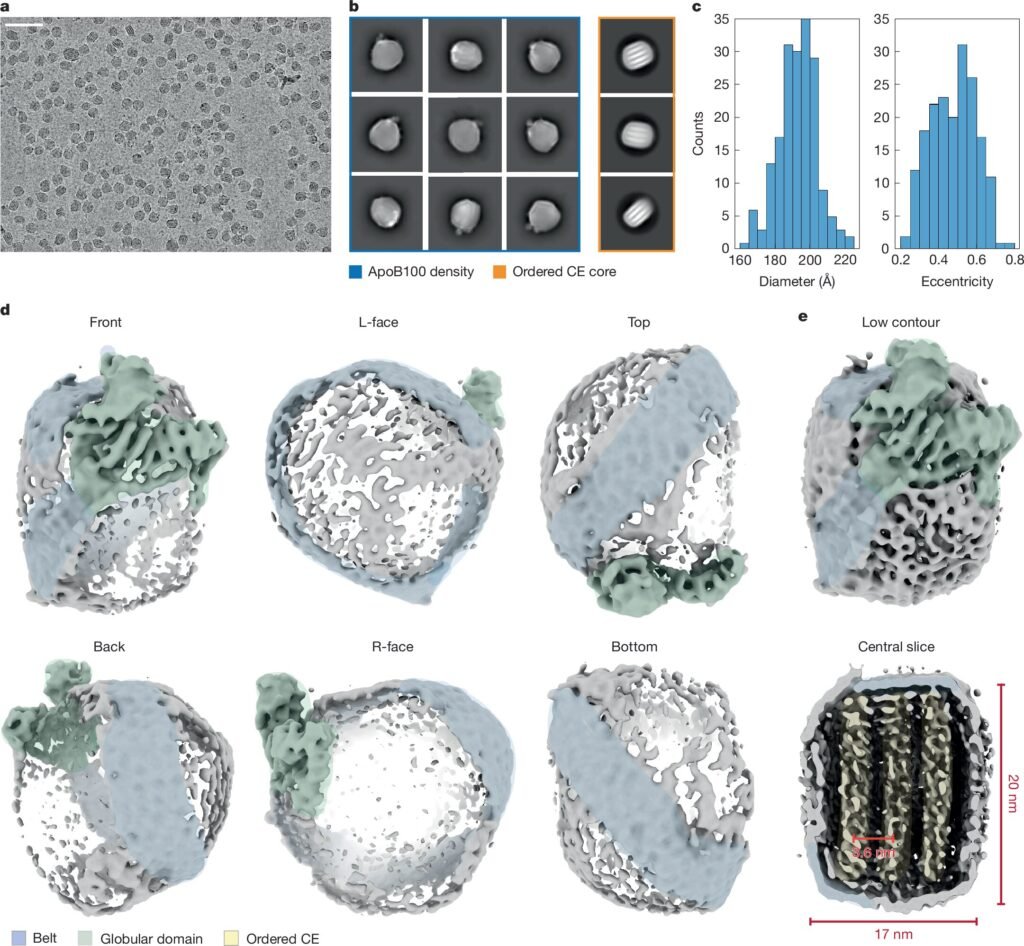
In a groundbreaking study published in Nature, researchers from the University of Missouri have unveiled the specific shape and structure of one of the body’s most complex proteins: ApoB100. This protein acts as a molecular exoskeleton, enveloping LDL particles and facilitating their journey through the bloodstream.
The implications of this discovery are significant. It paves the way for the development of new drugs that target LDL with greater precision, offering more effective treatments for high cholesterol and heart disease while potentially minimizing the side effects associated with current medications like statins.
The key to unraveling the mysteries of ApoB100 lay in cutting-edge technologies like cryo-electron microscopy. Researchers Zachary Berndsen and Keith Cassidy, experts in this field, utilized the state-of-the-art equipment at the Electron Microscopy Core within the Roy Blunt NextGen Precision Health building at Mizzou to achieve their breakthrough.
Cryo-electron microscopes provide a level of resolution far beyond traditional microscopes, allowing scientists to visualize the intricate structures of individual proteins on a minuscule scale. Berndsen and Cassidy leveraged this technology to uncover the structural secrets of ApoB100, shedding light on its role in cholesterol metabolism and heart disease.
Berndsen, a biochemist, used the cryo-electron microscope to determine the protein’s structure, while Cassidy, a physicist, employed artificial intelligence and Mizzou’s powerful supercomputers to create a detailed model of ApoB100. By integrating an AI neural network called AlphaFold with cryo-electron microscopy images, they achieved a higher-resolution understanding of the protein, opening new avenues for targeted treatments.
The significance of this research extends beyond the laboratory. By developing more precise tests that measure ApoB100 levels in the blood, researchers aim to provide a more accurate indicator of heart disease risk. For Berndsen and Cassidy, who both have a personal stake in the fight against heart disease, bridging the gap between basic science and practical health benefits is a driving force behind their work.
Ultimately, the discovery of ApoB100’s structure represents a crucial step towards improving our understanding of cholesterol metabolism and developing tailored treatments for heart disease. By leveraging cutting-edge technologies and interdisciplinary collaboration, researchers at the University of Missouri are at the forefront of scientific innovation with real-world impact.