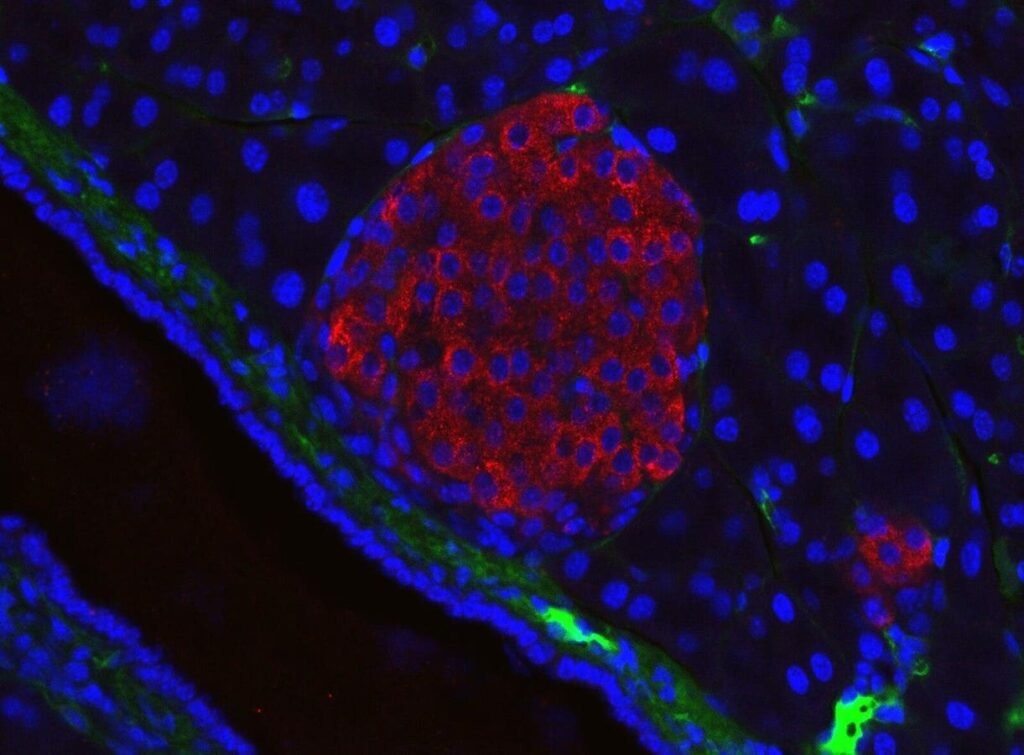
Mouse pancreatic islet visualised using immunofluorescent microscopy. — Colours: red = insulin antibody, blue = DAPI = nuclei, green unspecific anti-mouse secondary antibody (stains mostly intercellular matrix) Dimension: real width 326µm Generated in the Solimena lab, Paul Langerhans Institute Dresden
Jakob Suckale from Wikimedia Commons
For decades, the ambitious goal of regenerative medicine has been to replace damaged or lost tissues with healthy, functioning ones. Nowhere is this objective more compelling—or challenging—than in type 1 diabetes. Countless research efforts over the years have sought to restore these cells. Breakthroughs in cell engineering and transplantation are beginning to overcome long-standing roadblocks, and these recent advances are a significant achievement for regenerative medicine as a whole.
For millions living with the constant demands and worries of type 1 diabetes, this is more than promising data. It’s a moment that hints at a world where glucose monitors and needles might finally become relics of the past. With this, a life free from the daily burdens of diabetes suddenly feels not just possible, but imminent.
What Is Type 1 Diabetes?
Type 1 diabetes is a chronic autoimmune disorder defined by the immune system’s destruction of insulin-producing beta cells in the pancreas. Insulin is the hormone responsible for moving glucose from the bloodstream into the body’s cells for energy. Without it, blood sugar rises to dangerous levels. Over time, it can damage virtually every organ. Most people diagnosed are children or young adults, and living with type 1 diabetes requires ongoing vigilance: regular blood glucose testing, carefully calibrated insulin administration, and persistent risk of life-threatening hypoglycemia.
To survive, people with type 1 diabetes must monitor their blood sugar levels constantly. They frequently perform fingerstick tests throughout the day to check their blood glucose levels. Also, they must adjust their diet, exercise routine, and daily schedule to accommodate frequent insulin injections or an insulin pump. Despite advances in technology, keeping blood glucose within a safe range remains challenging, and the risk of life-threatening “hypoglycemic” episodes—when blood sugar drops too low—is ever-present.
Off-the-Shelf Stem Cell Therapy: Replacing What’s Lost
This is where the new therapies come into play. They go straight to the heart of the problem: the loss of the body’s own insulin-producing cells. Instead of requiring patients to rely on lifelong injections, the focus is now on developing stem cells grown in controlled laboratory environments. These stem cells are engineered to become fully functional islet cells. These are the very clusters that regulate blood sugar in a healthy pancreas.
The “off-the-shelf” nature of these cells is crucial. Earlier islet transplant procedures relied on donated organs, which are rare, variable in quality, and subject to lengthy waitlists. The engineered cells, on the other hand, are produced in large quantities. Also, they can be made available as needed. When infused, typically into the liver, these new cells integrate and begin responding to blood sugar levels in real time, releasing insulin as needed. In essence, the therapy is designed to restore the natural balance lost due to the disease. The promise of this approach is already becoming a reality.
Inside the Latest Study
The most recent results in the development of stem cell–derived therapies for type 1 diabetes stem from a clinical trial conducted by Vertex Pharmaceuticals. This study enrolled patients with established, severe type 1 diabetes. This is a group for whom current treatments often fail to prevent sudden and dangerous drops in blood sugar. Each patient in the trial received a single infusion of lab-grown islet cells, now referred to as zimislecel.
To translate the science: the stem cells are first turned into insulin-producing islet cells in the lab. These are then infused into the patient’s liver, not the pancreas. If successful, they begin sensing blood sugar levels and releasing insulin as needed, just like a healthy pancreas would. Think of this as swapping out a faulty part with a working one, rather than relying on external fixes.
The results over twelve months were consistent and dramatic. All participants in the study were found to be producing their own insulin again. This was confirmed by checking bloodwork to verify that the islet cells were now active. Therefore, the transplanted cells integrated well, and the patients’ bodies were able to produce insulin naturally for the first time in years. Even more remarkably, ten of these individuals were able to discontinue daily insulin injections entirely. Every participant met or surpassed the American Diabetes Association’s stringent targets for glycemic control. Notably, none suffered severe hypoglycemic episodes.
Why This Matters: The Science and Its Impact
These results represent a shift in the care landscape for type 1 diabetes. Previous attempts at islet cell replacement used donor cells, which were in very limited supply. By creating islets from universal stem cells, this therapy can be scaled up. It brings the possibility of universal therapies with consistent quality and availability. This offers real hope to millions worldwide. It also signals a foundational advance in regenerative medicine itself.
Demonstrating that we can produce, transplant, and integrate functional cell populations to cure a chronic, complex condition illustrates the practical power of stem cell technology. The methodology used here is already being adapted for other therapeutic targets: engineered heart muscle for cardiac repair, retinal cells for vision restoration, and dopaminergic neurons for Parkinson’s disease. It establishes a blueprint for tackling similar disorders and builds a bridge from bench research to real-world clinical impact.
These advances, however, are not without their complexities. Because the transplanted islet cells are not the patient’s own, recipients currently require immunosuppressive medications to prevent rejection—a challenge shared by all current forms of tissue and organ transplantation. Although initial safety profiles are encouraging and side effects manageable, ongoing studies are exploring methods to reduce or eliminate immune suppression, such as gene editing and immune cloaking techniques.
The Road Ahead: What’s Next?
The achievements described here are far more than incremental progress in diabetes care. They represent proof-of-concept for the broad ambitions of regenerative medicine: restoring lost tissue function, curing chronic diseases, and making transformative treatments widely accessible. As these strategies mature and diversify, their impact will almost certainly extend well beyond any single disease. They offer a pathway to fundamentally change how we approach a broad spectrum of degenerative and autoimmune conditions. The future of regenerative medicine, once hypothetical and distant, now approaches with real hope for curing—not simply managing—a host of devastating chronic conditions.