Antibodies have shown promise in improving the rehabilitation of individuals with acute spinal cord injuries. A recent study conducted by researchers at 13 clinics across Germany, Switzerland, the Czech Republic, and Spain has identified patient groups that have displayed a clinically relevant treatment effect. These findings have been published in The Lancet Neurology, with a follow-up study scheduled to commence in December 2024.
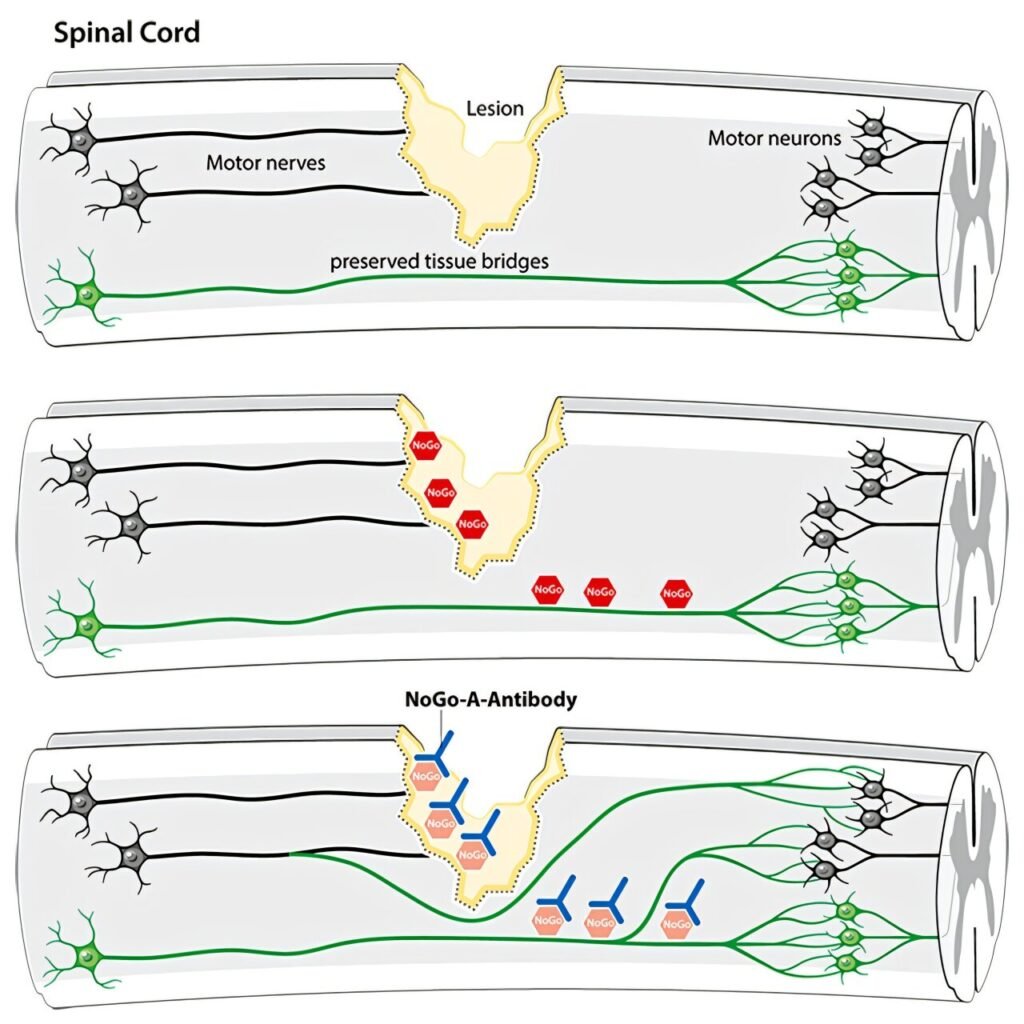
The study focused on the antibody NG 101 (anti-Nogo-A), which blocks the body’s Nogo-A protein, known to inhibit the regeneration of damaged nerve fibers in the spinal cord following an acute injury. By neutralizing this protein, the antibody aims to promote the regeneration of injured nerve tracts and facilitate spinal cord recovery.
A total of 126 participants between the ages of 18 and 70, all suffering from acute complete to incomplete spinal cord injuries in the neck region (tetraplegia), took part in the clinical trial. Seventy-eight individuals received the antibody via direct injection into the spinal canal, while 48 received a placebo. The treatment cycle consisted of six injections alongside comprehensive inpatient care.
The study, which was randomized, double-blind, and placebo-controlled, revealed significant improvements in patients with incomplete spinal cord injuries. These individuals showed enhanced voluntary activation of paralyzed muscles and increased functional independence in everyday tasks after six months of treatment. However, the treatment did not demonstrate significant improvements in patients with complete spinal cord injuries.
Overall, the antibody was well-tolerated with no reported side effects. The successful outcomes of this study, led by Balgrist University Hospital, highlight the potential of antibodies in rehabilitation for spinal cord injuries. While these initial results are promising, further studies are required to confirm the efficacy of the treatment. A follow-up study with an improved antibody is set to begin in December 2024, with patient subgroups selected based on anticipated responsiveness to the treatment.
For more information, the study has been published in The Lancet Neurology under the title “Safety and efficacy of intrathecal antibodies to Nogo-A in patients with acute cervical spinal cord injury: a randomised, double-blind, multicentre, placebo-controlled, phase 2b trial” (DOI: 10.1016/S1474-4422(24)00447-2). This research was conducted by Norbert Weidner et al. from the University of Zurich.
In conclusion, the use of antibodies in the treatment of acute spinal cord injuries shows promising results, offering hope for improved rehabilitation outcomes in affected individuals. Further research and clinical trials are essential to validate these findings and explore the full potential of antibody-based therapies in spinal cord injury rehabilitation.