AMPARs, or AMPA receptors, play a crucial role in fast excitatory neurotransmission in the brain, which is essential for learning and memory processes. These receptors are composed of different subunits, including GluA1, GluA2, GluA3, and GluA4, each contributing to their functional properties. Recent research conducted by McGill University has shed light on the varying degrees of calcium permeability exhibited by AMPARs based on their subunit composition.
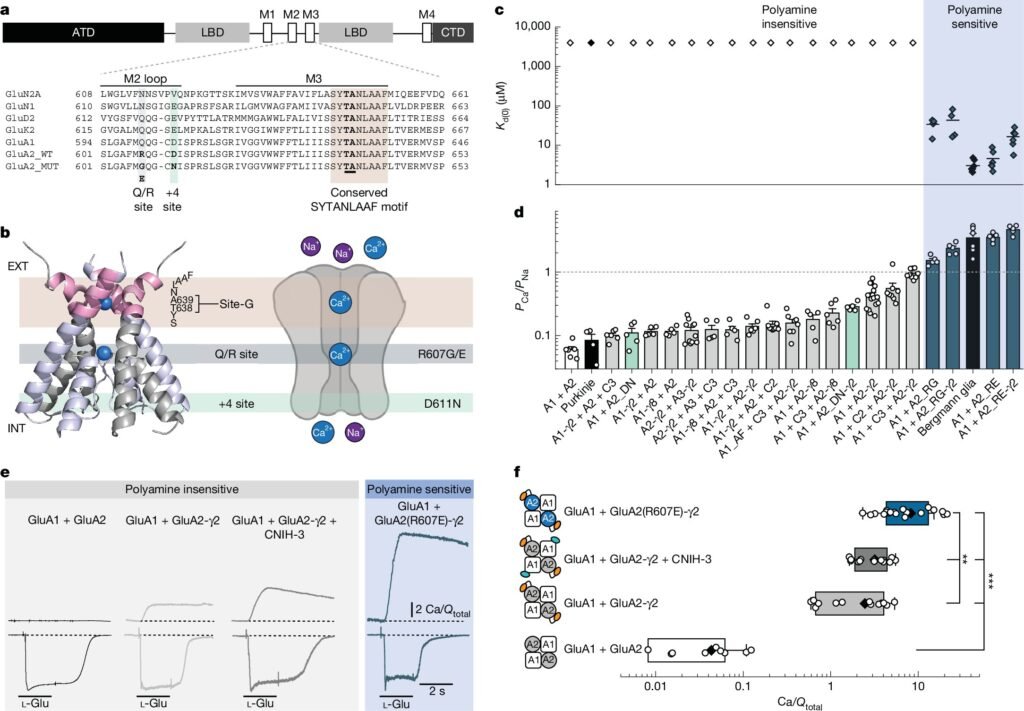
Traditionally, GluA2-containing AMPARs were believed to be calcium-impermeable due to RNA editing at the Q/R site, which replaced a glutamine with a positively charged arginine, blocking calcium permeability. However, the study unveiled that missense mutations in the GluA2 subunit, such as R607E and R607G, can modify calcium permeability. These mutations have been associated with neurodevelopmental disorders like autism and intellectual disability.
The research, published in Nature, demonstrated that GluA2-containing AMPARs form a continuum of calcium-permeable channels rather than fitting into a rigid binary classification. By reconstructing AMPARs with various auxiliary subunits to mimic their natural assembly in the brain, researchers were able to analyze calcium permeability using electrophysiology and calcium imaging techniques.
The study revealed that auxiliary proteins, particularly TARPs and CNIH proteins, significantly influenced calcium transport through AMPARs. Additionally, an extracellular calcium-binding site, known as Site-G, was identified as a key player in facilitating calcium entry into the receptor pore. These findings indicate that AMPARs operate along a spectrum of calcium permeability, challenging the traditional binary classification of these receptors.
Moreover, the research emphasized the impact of missense mutations in the GluA2 subunit on calcium permeability, highlighting the broader role of GluA2-containing AMPARs in calcium transport. These findings have implications for understanding neuronal communication and synaptic plasticity, showcasing the complexity and diversity of AMPAR function beyond previous assumptions.
In conclusion, the study at McGill University has provided valuable insights into the nuanced calcium permeability of AMPARs, emphasizing the need to revisit existing models and classifications. By uncovering the intricate mechanisms governing AMPAR function, this research paves the way for further exploration of the role of these receptors in brain function and neurological disorders.