Breast Milk IgG Shapes Neonatal Immune Responses to Gut Bacteria
A recent study conducted by Fred Hutchinson Cancer Center has shed light on the significant role of maternal immunoglobulin G (IgG) in shaping neonatal immune responses to gut bacteria in mice. The research found that IgG ingested during the first week of life played a crucial role in modulating microbiota-dependent adaptive immune responses even weeks after weaning. This early exposure to IgG was shown to fine-tune the immune system’s reactions to commensal microbes and dietary antigens.
The postnatal period is a critical phase for immune development in mammals, where the ability to properly adjust responses to new microbes and environmental antigens is vital for overall health. Breast milk is known to play a key role in shaping gut mucosal immunity by providing the infant gut with a range of components, including living microbes, oligosaccharides that select for beneficial bacteria, maternal cells, and immune mediators such as antibodies.
While previous research has primarily focused on the role of IgA in mucosal defense, the current study highlighted the abundance of IgG in breast milk and its impact on neonatal immunity. Published in Science, the study titled “Breast milk IgG engages the mouse neonatal immune system to instruct responses to gut antigens” aimed to dissect the specific functions and timing of breast milk antibodies during the postnatal period.
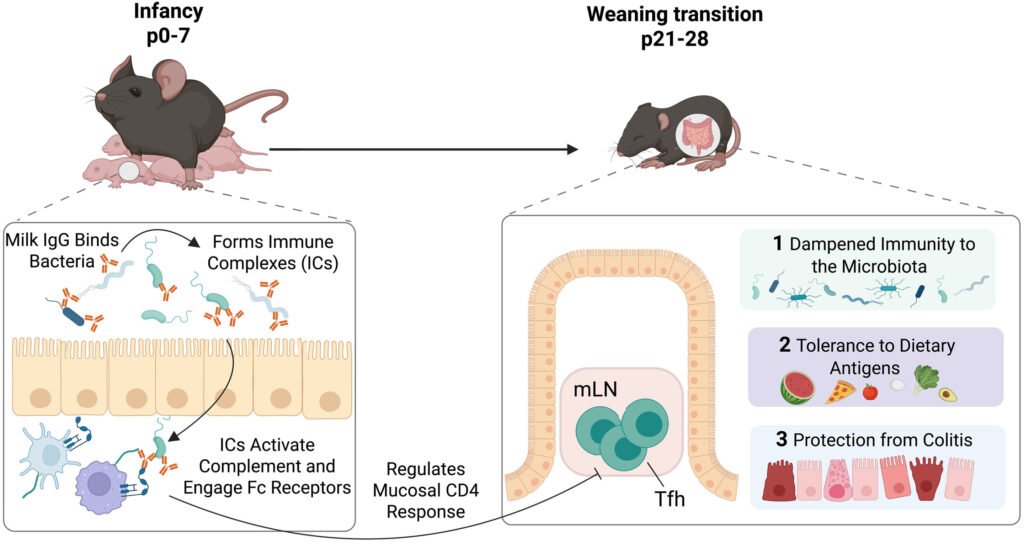
The researchers conducted experiments on newborn mice raised with or without maternal antibodies, with some pups being cross-fostered to control the timing of antibody exposure. Purified IgG from milk or blood was fed to the pups during the first week of life, and the presence of gut bacteria was manipulated using germ-free conditions and antibiotics. The study revealed that receiving maternal antibodies early in life helped prevent exaggerated immune reactions later on, as mice without early antibodies displayed overactive immune responses after weaning.
Interestingly, feeding purified IgG alone during the first week was sufficient to restore immune control, with specific subclasses of IgG binding strongly to bacteria in the neonatal gut. These IgG-microbe complexes activated neonatal antibody-sensing receptors and complement proteins, ultimately calibrating tolerance mechanisms that reduce the risk of inflammatory or allergic diseases. Mice that received early IgG were also found to be more resistant to colitis and had fewer allergic responses to dietary proteins.
The findings of this study underscore the active role of maternal IgG in instructing immune development during the critical postnatal period. By forming complexes with commensal bacteria in the neonatal gut, IgG helps shape long-term gut immune health and reduces the risk of inflammatory and allergic diseases. While the study was conducted in mice, the implications for human neonatal immunity are significant, highlighting the potential benefits of maternal antibodies in shaping immune responses in infants.
In conclusion, breast milk IgG emerges as a key player in calibrating neonatal immune responses to gut bacteria, providing vital instruction for the developing immune system. This research contributes to our understanding of the complex interplay between maternal antibodies and neonatal immunity, underscoring the importance of early-life immune modulation for long-term health outcomes.