A groundbreaking study led by Prof. Chen Chang from the Institute of Biophysics of the Chinese Academy of Sciences, in collaboration with Prof. Huang Zhangjian of China Pharmaceutical University, has shed light on a previously unknown molecular mechanism crucial for learning and memory.
Published in the journal Redox Biology, the research unveils the significance of S-nitrosation of CaMKIIα—a redox-based post-translational modification—in cognitive processes. The team also introduced SNOTAC, a precision S-nitrosation modulator distinct from traditional nitric oxide (NO) donors, offering a novel therapeutic avenue for memory impairment.
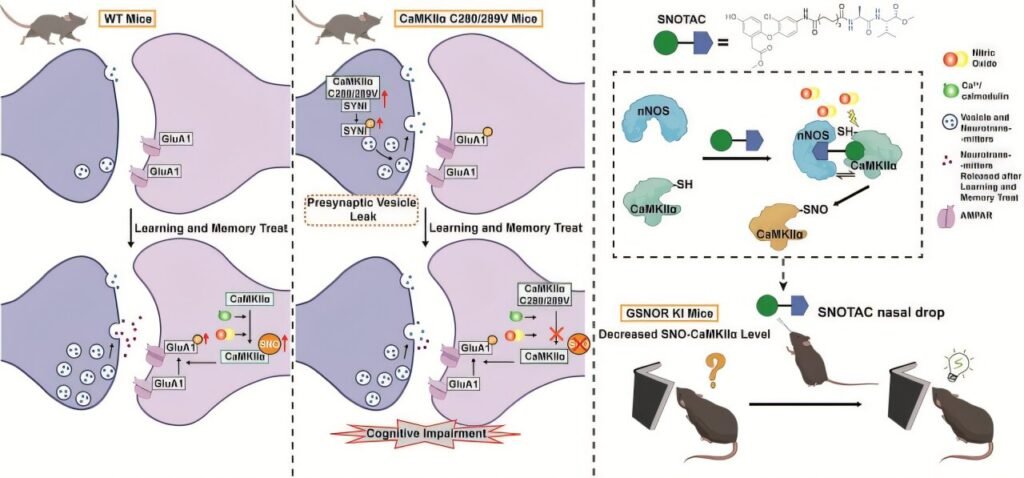
The investigation began by investigating changes in CaMKIIα S-nitrosation levels during physiological learning and memory activities. In experiments with wild-type mice, exposure to cognitive tasks significantly boosted CaMKIIα S-nitrosation in the hippocampus.
To assess the functional role of this modification, mutant mice that were unable to undergo S-nitrosation were generated. These mutant mice displayed notable deficits in learning and memory across various behavioral tests, indicating that CaMKIIα S-nitrosation is a crucial regulatory mechanism in cognitive function.
Further studies revealed that the absence of CaMKIIα S-nitrosation led to abnormally high presynaptic vesicle release at rest. During cognitive tasks, these mice exhibited reduced synaptic signaling capacity, ultimately impairing memory consolidation.
Building on Prof. Chen’s “precision redox” concept and the “5R” principles for antioxidant drug development, the team devised a targeted intervention strategy. They developed a small molecule “molecular glue” approach that brings the target protein into proximity with nitric oxide synthase (NOS), allowing for selective modification through S-nitrosation. This innovative strategy led to the creation of SNOTAC.
SNOTAC specifically brings neuronal nitric oxide synthase (nNOS) close to CaMKIIα, enhancing its S-nitrosation in a precise and selective manner. Administering SNOTAC intranasally successfully restored memory function in mice with deficits caused by brain-specific GSNOR overexpression.
This study marks the first demonstration of the critical role of CaMKIIα S-nitrosation as a post-translational modification in learning and memory, independent of the well-known phosphorylation pathway. It also highlights a novel mechanism in which abnormal presynaptic vesicle release contributes to cognitive decline, offering new insights into age-related memory loss.
The development of SNOTAC serves as a proof-of-concept for targeted redox modulation to rescue cognitive function, paving the way for future precision redox therapies. This research provides a promising avenue for addressing memory impairments and cognitive decline, offering hope for individuals struggling with cognitive deficits.