The quest to eliminate the need for brushing and flossing may soon become a reality, thanks to the groundbreaking research led by UC Berkeley’s Wenjun Zhang. Zhang, a professor of chemical and biomolecular engineering, is on a mission to identify the beneficial bacteria in our mouths and enhance their presence to create a probiotic oral microbiome that can combat cavities.
The mouth’s microbiome is a diverse ecosystem of bacteria, with some species contributing to plaque formation and tooth decay. Traditional studies have focused on identifying specific bacteria associated with cavities, but Zhang’s approach takes it a step further by analyzing the entire DNA sequence of the oral bacteria to pinpoint gene clusters linked to cavity formation.
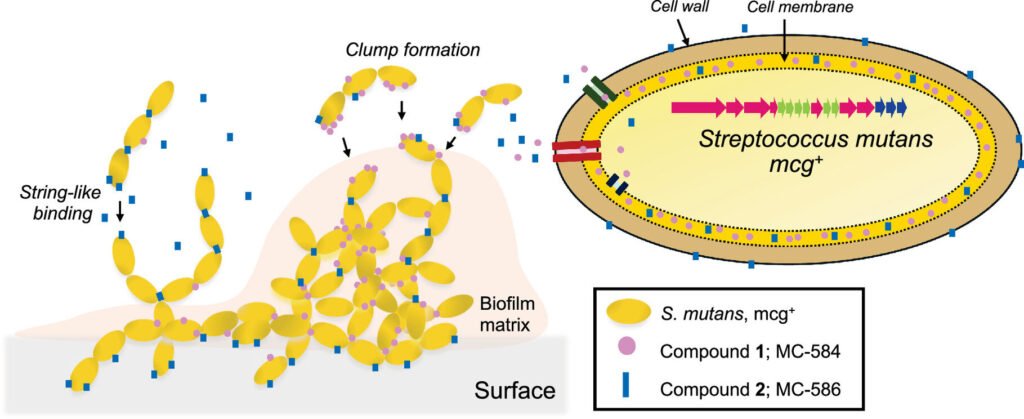
In a recent publication in the journal Proceedings of the National Academy of Sciences, Zhang and her team unveiled a gene cluster responsible for producing two molecules that enable bacteria to stick together and form a robust biofilm on teeth. This gene cluster, found in certain strains of cavity-causing bacteria like Streptococcus mutans, presents an opportunity to introduce these molecules to beneficial bacteria, enhancing their ability to adhere to teeth and outcompete harmful bacteria.
The discovery of these specialized metabolites, named mutanoclumpins, opens up new possibilities for promoting oral health. By understanding how these molecules influence biofilm formation, researchers could develop genetic or chemical inhibitors to prevent cavity-causing bacteria from producing them. Additionally, enhancing the biofilm-forming capabilities of beneficial bacteria such as Streptococcus salivarius could optimize their probiotic potential.
The research conducted by Zhang’s team, including graduate student McKenna Yao, sheds light on the significance of these secondary metabolites in the human microbiome. By unraveling the complex interactions within the oral microbiome, researchers hope to pave the way for innovative strategies to prevent cavities and promote oral health.
Moving forward, the team plans to map out the diverse array of specialized metabolites produced by oral bacteria to gain a comprehensive understanding of the oral microbiome’s dynamics. While brushing remains the most effective method for removing biofilm, the potential for disrupting biofilm formation through targeted interventions offers a promising avenue for future research.
The collaborative effort of researchers like Zhang, Yao, and their colleagues underscores the importance of exploring the untapped potential of the human microbiome in enhancing overall health. By harnessing the power of beneficial bacteria and specialized metabolites, we may soon bid farewell to cavities and usher in a new era of oral health.