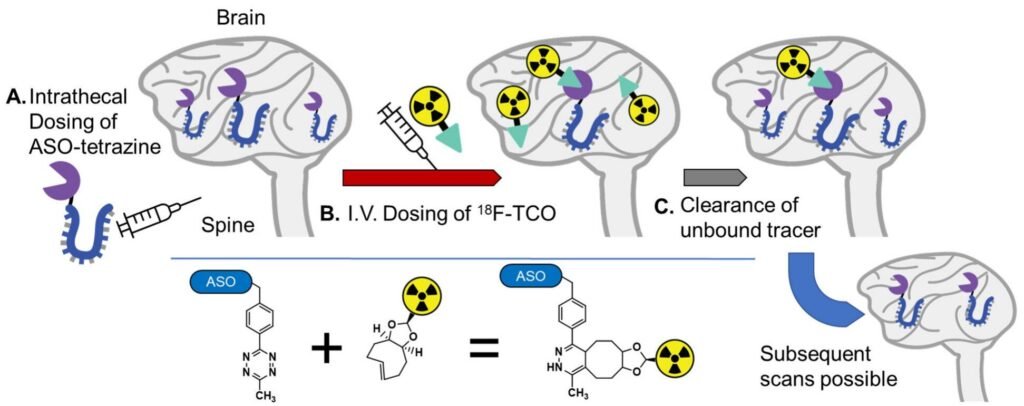
The innovative approach involves the use of a PET tracer that binds to the antisense oligonucleotide in the brain, allowing for precise imaging of the medication’s distribution. This method, known as pretargeted imaging, involves administering the antisense oligonucleotide conjugate directly into the brain and then injecting the PET tracer intravenously. The PET tracer crosses the blood-brain barrier and binds specifically to the antisense oligonucleotide in the brain, while unbound tracer is cleared, enabling clear visualization of the medication’s distribution through PET imaging.
The study demonstrates the feasibility of using click chemistry to track the biodistribution of antisense oligonucleotides in the brain, offering new insights into how these medications interact with neural tissue. This information is crucial for optimizing treatment strategies for neurological disorders and ensuring that medications reach their intended targets effectively.
The potential of antisense oligonucleotides as a therapeutic approach for neurological disorders is vast, and this research paves the way for further advancements in the field. By utilizing Nobel prize-winning click chemistry techniques, scientists are able to overcome the challenges associated with assessing the distribution of these medications in the brain, bringing us one step closer to more effective treatments for conditions like Parkinson’s and Alzheimer’s diseases.
The collaboration between researchers in the United States and Sweden highlights the importance of international cooperation in advancing scientific knowledge and developing innovative solutions to complex medical problems. With continued research and development in this area, we may soon see groundbreaking treatments that harness the power of antisense oligonucleotides to combat devastating neurological conditions. The newly developed tracer molecule, [18F]BIO-687, was used to track the distribution of modified antisense oligonucleotides in the body. This tracing molecule allowed researchers to visualize how the antisense oligonucleotides interacted with specific targets in the body, providing valuable insights into the effectiveness of the modified compounds.
Using PET imaging technology, Cook and his team were able to monitor the movement of the antisense oligonucleotides in real-time, tracking their distribution and accumulation in different tissues and organs. This groundbreaking methodology has the potential to revolutionize the field of molecular imaging, offering new possibilities for studying the mechanisms of action of therapeutic compounds and developing more targeted treatment strategies.
The use of click chemistry in combination with PET imaging represents a powerful tool for researchers in the field of molecular biology and pharmacology. By enabling the visualization of specific molecular interactions in living organisms, this technique opens up new avenues for studying disease processes, drug delivery mechanisms, and therapeutic responses.
The awarding of the Nobel Prize in Chemistry to Carolyn Bertozzi, Morten Meldal, and Karl Barry Sharpless highlights the importance of click chemistry in advancing the field of molecular imaging and drug development. Their groundbreaking work has paved the way for innovative research like Cook’s PET imaging methodology, which promises to impact the future of medicine and healthcare.
Overall, Cook’s specialization in click chemistry and tracers for PET scanning technology represents a significant advancement in the field of molecular imaging. By developing new methodologies for tracking and visualizing molecular interactions in the body, researchers are able to gain a deeper understanding of biological processes and create more effective treatments for a wide range of diseases.