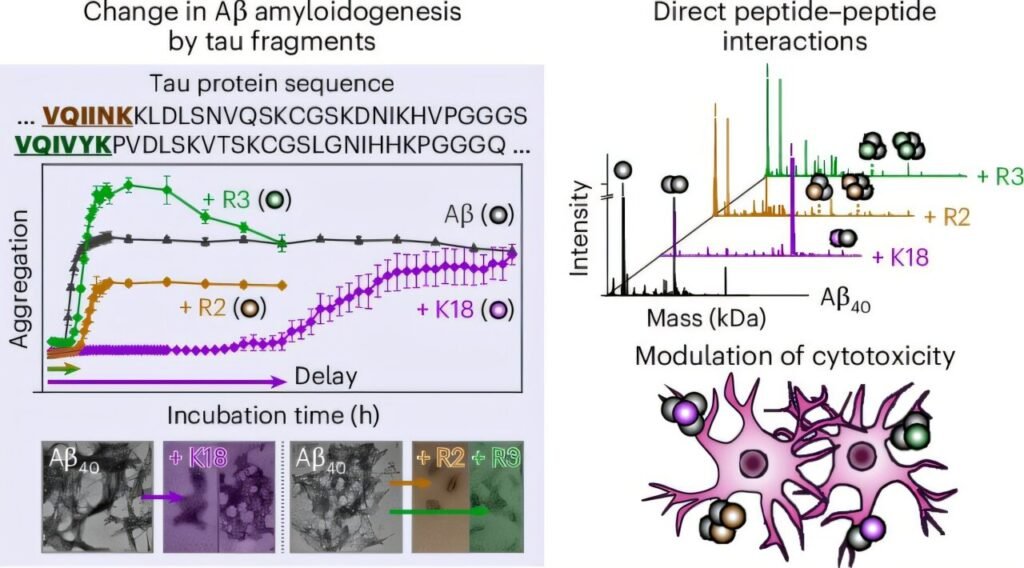
A groundbreaking study conducted by a Korean research team has shed light on the communication between tau and amyloid-β proteins in Alzheimer’s disease. With an estimated 50 million people worldwide suffering from dementia, Alzheimer’s disease remains a predominant neurodegenerative brain disorder. The research, published in the journal Nature Chemical Biology, unveils a molecular-level understanding of how these two key pathological proteins interact to regulate toxicity.
Led by Professor Mi Hee Lim from the Department of Chemistry at the Korea Advanced Institute of Science and Technology (KAIST), the study has uncovered that the microtubule-binding domain of tau directly interacts with amyloid-β, altering its aggregation pathway and reducing cellular toxicity. Tau and amyloid-β are known for forming neurofibrillary tangles and amyloid plaques, respectively, in the brains of Alzheimer’s patients.
While these proteins typically accumulate in different regions of the brain, the research team discovered that specific regions of tau can bind to amyloid-β, forming tau-amyloid-β heterocomplexes. This interaction shifts amyloid-β towards a less toxic aggregation pathway, ultimately reducing the toxicity associated with Alzheimer’s disease. By delaying the nucleation stage of amyloid aggregation, tau plays a crucial role in mitigating the progression of the disease.
Utilizing advanced analytical techniques, the team elucidated the structural, thermodynamic, and functional properties of tau-amyloid interactions. The study revealed that the hydrophilic and hydrophobic characteristics of tau’s microtubule-binding repeats influence its binding affinity with amyloid-β. These findings mark a significant advancement in understanding the molecular mechanisms underlying dementia and other neurodegenerative disorders.
Dr. Young-Ho Lee of the Korea Basic Science Institute emphasized the importance of multidisciplinary research in unraveling the complexities of neurodegenerative diseases. The study’s findings open new avenues for therapeutic targets not only in Alzheimer’s disease but also in other protein aggregation-based disorders.
Professor Mi Hee Lim highlighted the significance of the study in identifying new molecular motifs that could revolutionize the treatment of neurodegenerative brain disorders. The research provides valuable insights into the pathological understanding of Alzheimer’s disease and offers hope for the development of novel therapies.
In conclusion, the study’s findings pave the way for future research into the molecular interactions and protein aggregation processes involved in neurodegenerative diseases. By uncovering the intricate relationship between tau and amyloid-β proteins, this research holds promise for advancing the diagnosis and treatment of Alzheimer’s and other related disorders.