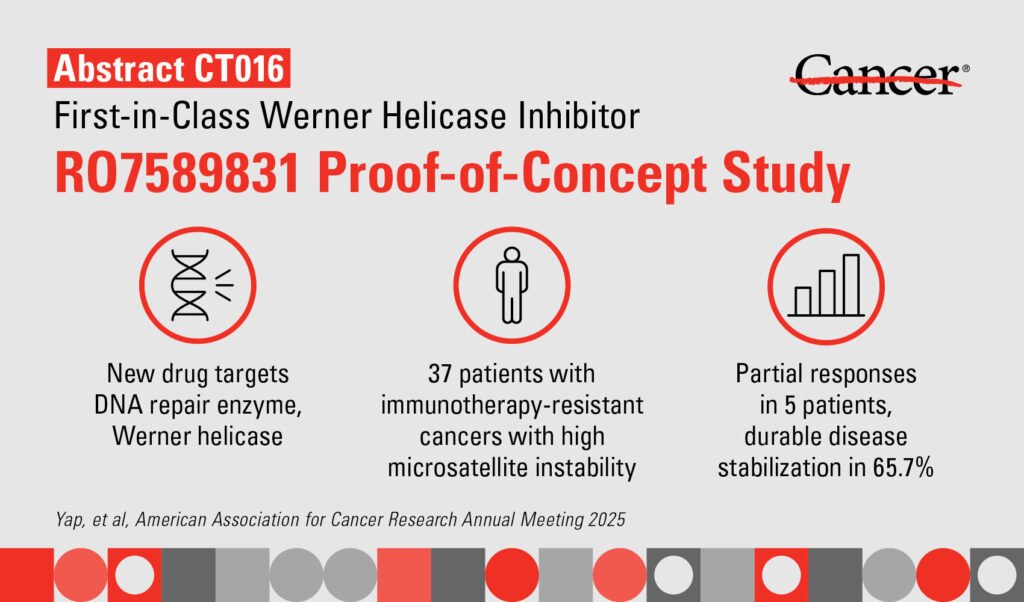
Targeted therapy has shown promising results in a Phase I trial for patients with certain genetic defects. The therapy, known as RO7589831, targets the DNA repair enzyme Werner helicase and has demonstrated early signs of efficacy while being well-tolerated. These findings were reported by researchers at The University of Texas MD Anderson Cancer Center.
Presented at the American Association for Cancer Research (AACR) Annual Meeting 2025, the results of the trial were shared by principal investigator Timothy Yap. Yap emphasized the safety and potential efficacy of RO7589831, highlighting the importance of having treatment options for patients with limited choices. The therapy targets Werner helicase, an enzyme crucial for the survival of many cancers, making it an exciting breakthrough in the field.
RO7589831 belongs to a new class of drugs that focus on the DNA damage response in patients with solid tumors and specific genetic mutations like high microsatellite instability (MSI) or deficient mismatch repair (dMMR). These mutations are present in a significant percentage of patients with solid tumors who may not respond well to conventional treatments like immune checkpoint inhibitors.
By inhibiting Werner helicase, RO7589831 disrupts DNA repair in tumor cells, leading to their death while sparing normal cells. The trial showed promising results with partial responses in multiple cancer types and lasting stable disease in a majority of patients. Metabolic imaging revealed correlations between metabolic responses and disease control, indicating the therapy’s effectiveness.
In terms of safety, most patients experienced mild adverse events, with nausea, vomiting, and diarrhea being the most common. No dose-limiting toxicities were observed, and ongoing trials are working to establish the optimal recommended dose for future studies.
Funded by Roche, this groundbreaking trial offers hope for patients with challenging genetic mutations. With further research and development, targeted therapies like RO7589831 could provide new treatment options for individuals with limited choices. For more information and a detailed list of authors and disclosures, the abstract of the study can be accessed online.
This article was originally published by the University of Texas MD Anderson Cancer Center and is subject to copyright. For more information and to access the original content, visit the provided link to the University’s website.