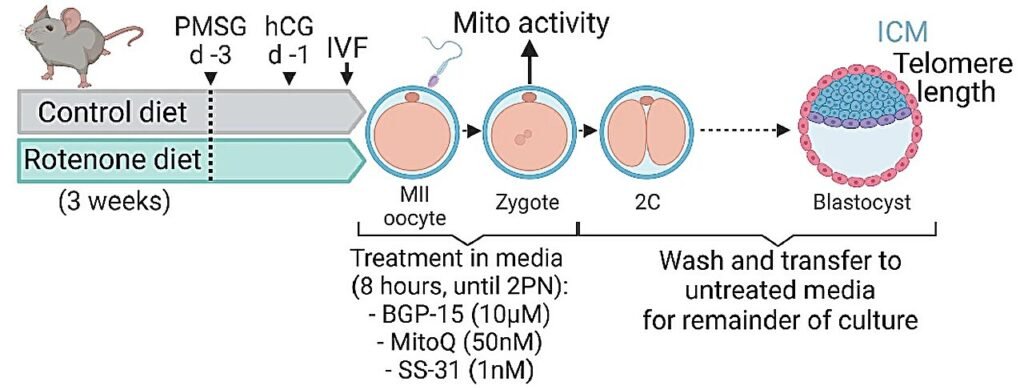
Researchers at the University of Adelaide have made a groundbreaking discovery that sheds light on how the earliest stages of embryo development can impact an individual’s future health and aging. The team, led by Professor Rebecca Robker, found that cellular processes within the egg at the time of fertilization play a crucial role in determining the length of telomeres in offspring.
Telomeres are protective caps at the ends of chromosomes that influence tissue growth and rejuvenation. Babies born with shorter telomeres are at a higher risk of developing chronic diseases associated with aging later in life. Factors such as maternal health and environmental conditions at the time of conception can influence telomere length and the offspring’s susceptibility to age-related diseases.
The research, published in Nature Communications, also revealed that it is possible to reverse cellular damage and restore telomere length in embryos using specific pharmaceutical compounds. This breakthrough opens up new possibilities for therapies in reproductive medicine and fertility treatments.
The study highlights the importance of focusing on women’s health and well-being in public health policies. By understanding the link between early embryo development and long-term health outcomes, researchers hope to develop interventions that can optimize cellular biology and reduce the risk of chronic diseases.
Moving forward, the team is collaborating with Vitaleon Pharma to further develop these findings into practical therapies for use by fertility specialists. By targeting mitochondrial-nuclear communication at fertilization, researchers aim to pave the way for personalized medicine approaches that can improve overall health and well-being.
This research underscores the critical role that early embryo development plays in shaping healthy lifestyles and emphasizes the need for tailored interventions to support maternal health and improve outcomes for future generations.