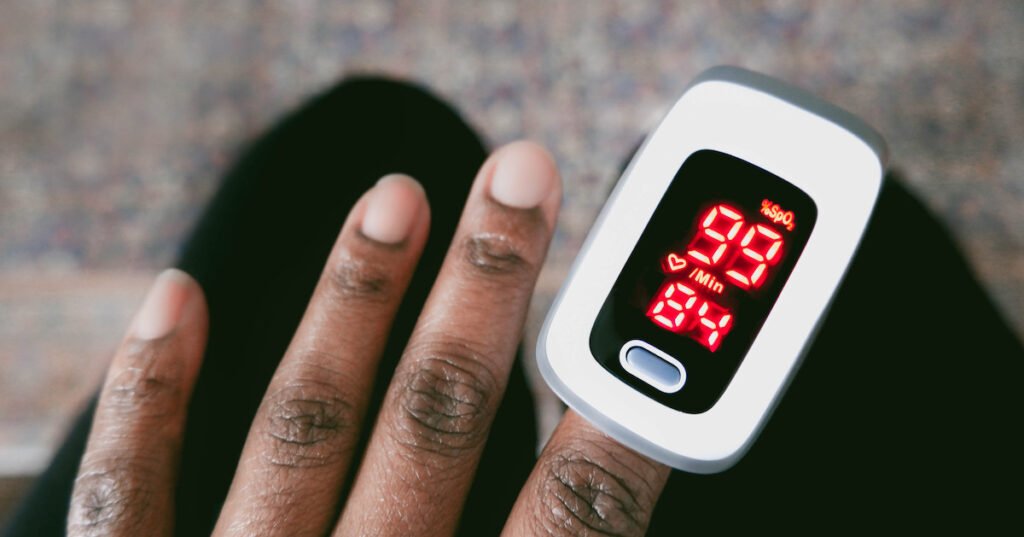
The U.S. Food and Drug Administration is inviting the public to provide feedback on updated recommendations for nonclinical and clinical performance testing for pulse oximeters used for medical purposes. These devices are designed to estimate the oxygen levels in arterial blood and monitor pulse rate.
WHY IT MATTERS
In light of the COVID-19 pandemic, concerns arose regarding the accuracy of pulse oximeters, particularly for individuals with darker skin tones. In response to these issues, the FDA issued a warning in 2023 about the potential inaccuracies of pulse oximeters in certain circumstances, highlighting that devices may be less reliable for individuals with dark skin pigmentation. Subsequent public meetings were held to reevaluate existing guidance on pulse oximetry.
The newly proposed update, titled "Pulse Oximeters for Medical Purposes – Non-Clinical and Clinical Performance Testing, Labeling and Premarket Submission Recommendations," aims to enhance the evaluation of device performance accuracy across different skin pigmentations. The guidance emphasizes the importance of collecting real-world and laboratory clinical data using both subjective and objective methods to standardize the assessment of pulse oximeter products used in healthcare settings.
According to the FDA, the updated recommendations apply specifically to pulse oximeters intended for medical use in hospitals or medical offices. Some devices currently on the market may already meet the revised performance criteria without requiring significant hardware or software modifications. In cases where a sponsor submits updated labeling to demonstrate comparable performance across skin pigmentations, the FDA commits to expediting the review process to ensure timely access to safe and accurate pulse oximeters.
It’s important to note that the updated guidance does not extend to general wellness products or devices for sporting/aviation purposes that are not subject to FDA review.
THE LARGER TREND
Research published in the New England Journal of Medicine in 2020 highlighted disparities in pulse oximeter accuracy between Black and white patients, with Black individuals being more likely to experience occult hypoxemia. Criticism has been raised regarding the FDA’s regulation of pulse oximeters, particularly in the context of the agency’s response time and review processes.
In response to these concerns, the FDA has been collaborating with stakeholders and clinical researchers to inform the update of pulse oximetry guidance. Efforts to address systemic racism in healthcare, improve patient safety, and enhance the reliability of pulse oximeters are ongoing within the medical device industry.
ON THE RECORD
Dr. Michelle Tarver, Director of the FDA’s Center for Devices and Radiological Health, emphasized the agency’s commitment to promoting the development of high-quality and effective medical devices. The draft recommendations aim to address disparities in pulse oximeter performance based on skin pigmentation, reflecting the FDA’s dedication to evidence-based decision-making.
For more healthcare IT news and updates, contact Senior Editor Andrea Fox at afox@himss.org. Healthcare IT News is a publication of HIMSS Media.
By incorporating these updates into a WordPress platform, healthcare professionals and stakeholders can stay informed about the evolving landscape of pulse oximetry guidelines and advancements in medical device technology.