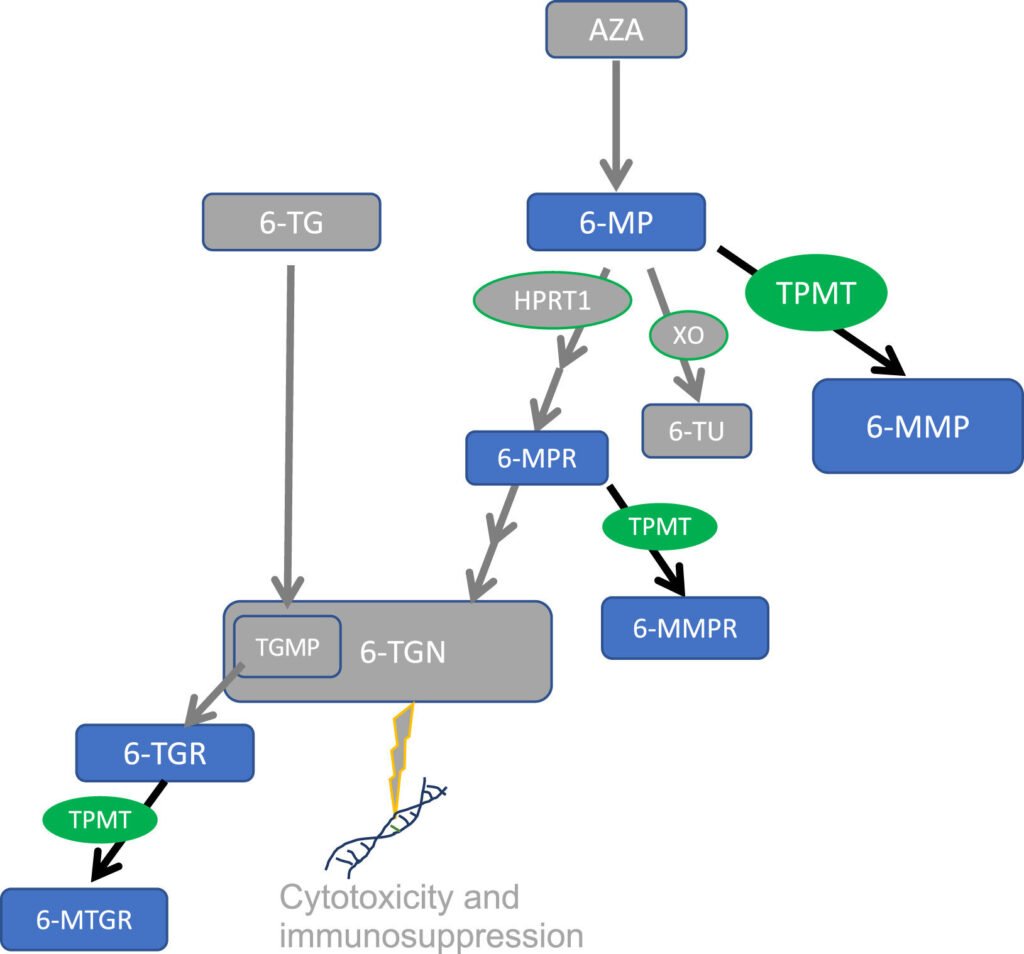
Researchers at the Mayo Clinic have made a significant discovery regarding a gene variant associated with reduced metabolism of thiopurine drugs. The TPMT∗8 allele, commonly found in individuals of African or African American descent, has been linked to decreased metabolism of thiopurines, which are used to treat various medical conditions such as leukemia, inflammatory bowel disease, and autoimmune disorders.
Published in The Journal of Molecular Diagnostics, the study aims to provide pharmacogenomic testing for predicting drug responses across all patient backgrounds. The research focused on the TPMT enzyme, crucial for metabolizing thiopurine medications and regulating active metabolite levels to prevent toxicity.
Lead author Dr. Rosalie M. Sterner from the Mayo Clinic in Rochester explained that variants in the TPMT gene can lower enzyme activity, leading to adverse reactions to thiopurine treatment. While current guidelines do not recommend testing for the TPMT∗8 variant due to its uncertain function, the study shed light on its impact on drug metabolism.
Combining data from TPMT activity and genotyping assays, the researchers compared individuals carrying the TPMT∗8 allele to those with normal TPMT function. They found that TPMT∗8 carriers metabolized 6-mercaptopurine slower than normal individuals but faster than intermediate metabolizers, affecting toxicity levels.
Co-lead investigator Dr. Ann M. Moyer noted the importance of TPMT activity in drug metabolism and toxicity, emphasizing the need for comprehensive genotyping through next-generation sequencing to detect all relevant alleles across different ancestral groups.
The study highlighted the potential clinical implications of reduced function alleles like TPMT∗8, suggesting the need for further research to determine appropriate dosing adjustments and incorporation into dosing guidelines. Dr. Sterner emphasized the importance of understanding the impact of reduced function alleles on drug tolerance and the potential for personalized medicine approaches.
These findings have the potential to shape clinical practice by improving phenotype prediction and guiding dosing strategies for thiopurine medications. As research in pharmacogenomics advances, the inclusion of diverse genetic backgrounds in testing and treatment recommendations will be crucial for optimizing patient care.