Studying the early development of human embryos is a complex task due to ethical restrictions and technical challenges. As a result, researchers often turn to animal models to gain insights into the biological processes that govern embryo development before implantation in the uterus.
A recent study conducted by researchers at Karolinska Institutet in collaboration with Université de Montréal has shed light on the potential of guinea pigs as an alternative model for studying this critical period. The research has revealed that guinea pig embryos exhibit several important similarities with human embryos, offering new avenues for exploration in fertility and stem cell biology.
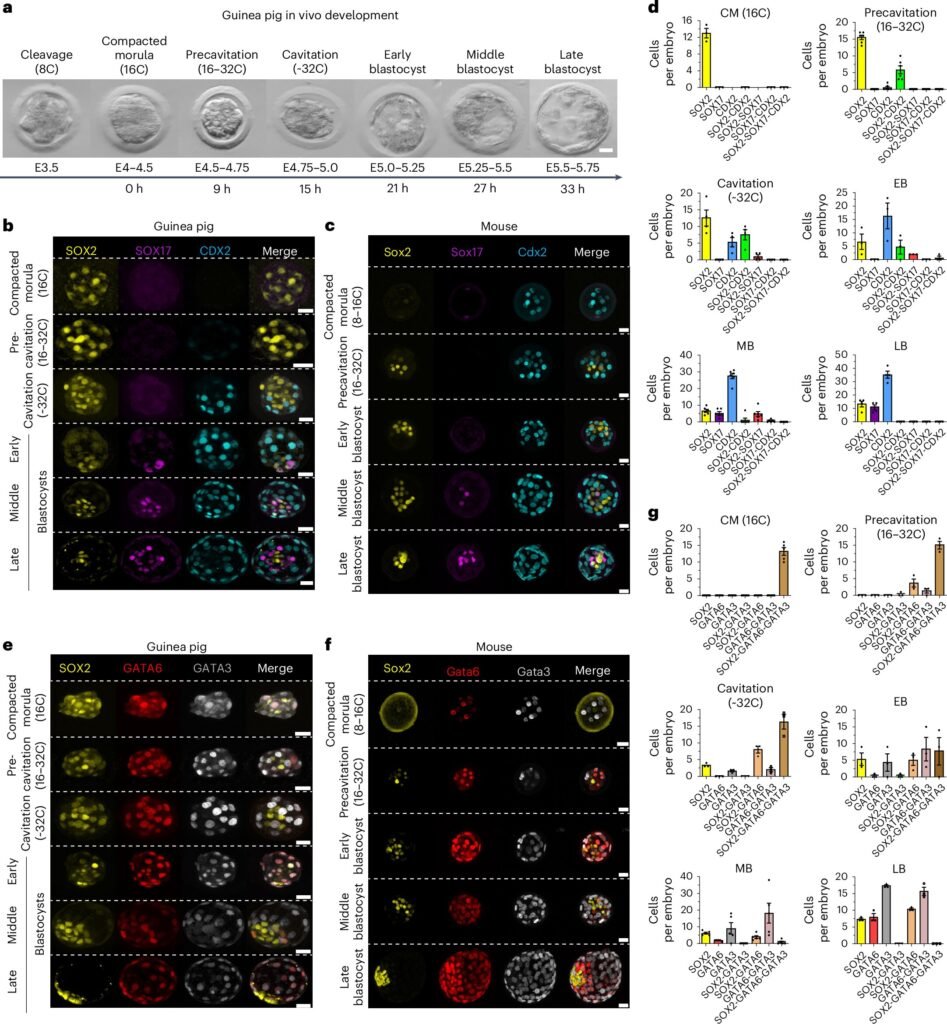
The study, published in Nature Cell Biology, utilized advanced single-cell RNA sequencing to analyze gene expression patterns during guinea pig embryo development. The results demonstrated that the preimplantation phase of guinea pig embryos, prior to uterine attachment, closely resembles that of humans. Additionally, the researchers identified similarities in the mechanisms regulating embryo attachment, including the involvement of retinoic acids and the nuclear receptor NR2F2. This discovery holds promise for enhancing our understanding of implantation failures in humans and potentially developing strategies to prevent them.
Lead author Sophie Petropoulos from the Department of Clinical Science, Intervention, and Technology at Karolinska Institutet highlighted the parallels between guinea pig and human embryo development, emphasizing the shared timing of cell specialization and gene regulation processes. The study also uncovered conserved pathways such as the Hippo, MEK-ERK, and JAK-STAT systems, which play crucial roles in cell differentiation during embryonic development in both species.
Unlike traditional laboratory animals like mice, guinea pigs exhibit a preimplantation development timeline and molecular circuitry that more closely resemble those of humans. Furthermore, both guinea pigs and humans undergo a similar type of implantation process, distinct from rodents like mice. Guinea pigs also possess a placental structure akin to humans, allowing researchers to investigate how early environmental influences, such as chemical exposure, impact embryo development and future health outcomes.
Petropoulos emphasized the potential of guinea pigs as a valuable model for studying the long-term effects of disturbances in embryonic development on health. With guinea pigs offering a more accurate reflection of human development compared to other animal models, researchers can gain valuable insights into the impact of the embryonic environment on future health outcomes. This knowledge could pave the way for advancements in assisted reproduction techniques and a deeper understanding of developmental disorders.
The researchers anticipate that the use of guinea pigs as a model organism will not only enhance research in assisted reproduction but also provide valuable insights into stem cell biology and regenerative medicine. By leveraging the unique characteristics of guinea pig embryos, future studies can contribute to the development of innovative treatments and interventions in the field of reproductive health.
In conclusion, the study underscores the potential of guinea pigs as a valuable model for studying early embryonic development and highlights the importance of utilizing diverse animal models to unravel the complexities of human biology. The findings offer a promising outlook for future research endeavors in fertility, stem cell biology, and developmental disorders.