Cardiac regeneration is a complex process that has the potential to revolutionize the treatment of heart failure. A recent study conducted by Professor Kai-Chien Yang’s group at National Taiwan University has shed light on the role of N-Cadherin, an adherens junction protein, in promoting cardiac regeneration.
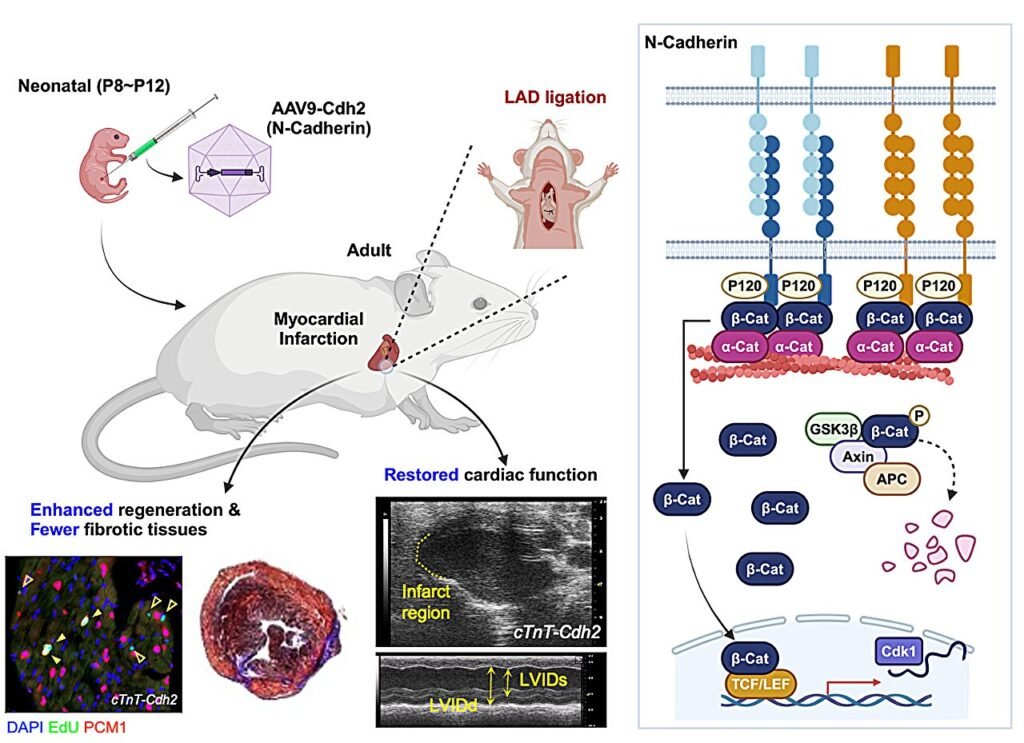
The research, published in Nature Communications, highlights the importance of N-Cadherin in regulating cardiomyocyte proliferation and renewal. The study found that N-Cadherin levels decrease with age, correlating with a decline in mitotic gene activity. In neonatal mice, injury triggers an increase in N-Cadherin expression, leading to enhanced cardiomyocyte mitosis.
Further experiments revealed that manipulating N-Cadherin levels can significantly impact cardiomyocyte proliferation. Decreasing N-Cadherin expression resulted in reduced cell division, while overexpressing it led to increased proliferation. Mechanistic studies demonstrated that N-Cadherin interacts with β-Catenin, a pro-mitotic transcription regulator, to drive cardiomyocyte self-renewal.
Moreover, targeted deletion of N-Cadherin in cardiomyocytes impaired cardiac regeneration in neonatal mice, causing excessive scarring. Conversely, overexpressing N-Cadherin promoted regeneration in adult mouse hearts following ischemic injury. These findings suggest that targeting N-Cadherin could be a promising strategy for enhancing cardiac regeneration and restoring function in injured adult human hearts.
The potential therapeutic implications of this research are immense, offering hope for improving outcomes for heart failure patients. By understanding the role of N-Cadherin in cardiac regeneration, researchers may be able to develop new treatments that harness the regenerative potential of the heart.
For more information, the study titled “N-Cadherin promotes cardiac regeneration by potentiating pro-mitotic β-Catenin signaling in cardiomyocytes” can be found in Nature Communications. This groundbreaking research paves the way for future advancements in the field of cardiac regeneration and holds promise for the development of novel therapies for heart failure patients.
This article was originally published by National Taiwan University and can be accessed on their website for further details.