In a groundbreaking study by researchers from Japan, a team led by Professor Naoyuki Inagaki from Nara Institute of Science and Technology has uncovered a crucial molecular mechanism involving a protein called shootin1b that drives the rapid movement of cells in glioblastoma, a deadly brain tumor. This discovery sheds light on the intricate machinery behind cell migration and the potential implications for cancer treatment.
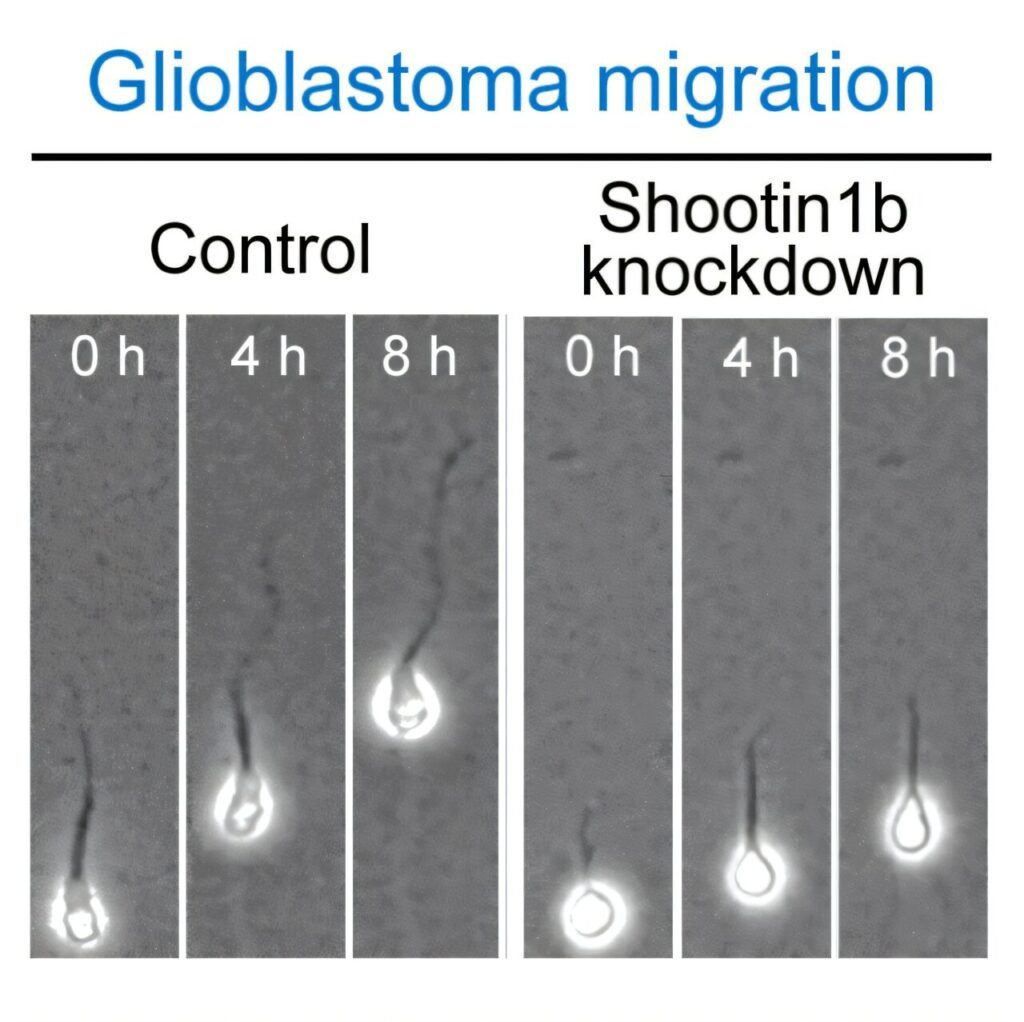
Glioblastoma is known as one of the most challenging brain tumors to treat, with a dismal five-year survival rate of only 5%. Understanding the molecular interactions that fuel the spread of cancer cells is essential for developing effective therapies. The abnormal activity of shootin1b was found to promote the migration of glioblastoma cells, highlighting its role as a key player in driving cancer progression.
The study revealed that shootin1b forms clutches that connect the cell’s internal actin to the external environment through adhesive molecules. This mechanism converts the backward movement of actin into traction force, propelling the cell forward. By targeting shootin1b and inhibiting its abnormal activity, researchers were able to prevent the migration of glioblastoma cells, offering a potential therapeutic strategy for combating this aggressive cancer.
Furthermore, the research identified a newly discovered molecular interaction that drives the rapid migration of dendritic cells, immune cells that play a crucial role in capturing pathogens. Understanding how shootin1b influences the movement of both cancer cells and immune cells provides valuable insights into the complex dynamics of cell migration in health and disease.
The findings from this study, published in Advanced Science, have significant implications for the development of novel treatment approaches for glioblastoma. By targeting shootin1b and disrupting its role in promoting cell migration, researchers hope to pave the way for more effective therapies that can improve the outcomes for patients battling this challenging form of brain cancer.
Overall, this research underscores the importance of understanding the molecular mechanisms that drive cell migration in cancer and highlights the potential of targeting shootin1b as a promising therapeutic strategy for inhibiting the spread of glioblastoma. With further investigation and development, these findings could lead to new treatment options that offer hope to patients facing this devastating disease.