Cell death is a crucial process in the life cycle of multicellular organisms, just as cell division is. Programmed cell death, also known as apoptosis, is essential for the proper development and functioning of the body. In recent years, a new form of programmed cell death called ferroptosis has emerged as a promising target for cancer treatment. This type of cell death is mediated by iron molecules and involves the oxidation of phospholipids in the cell membrane. However, some cancer cells have shown resistance to ferroptosis, which poses a challenge for developing effective therapies.
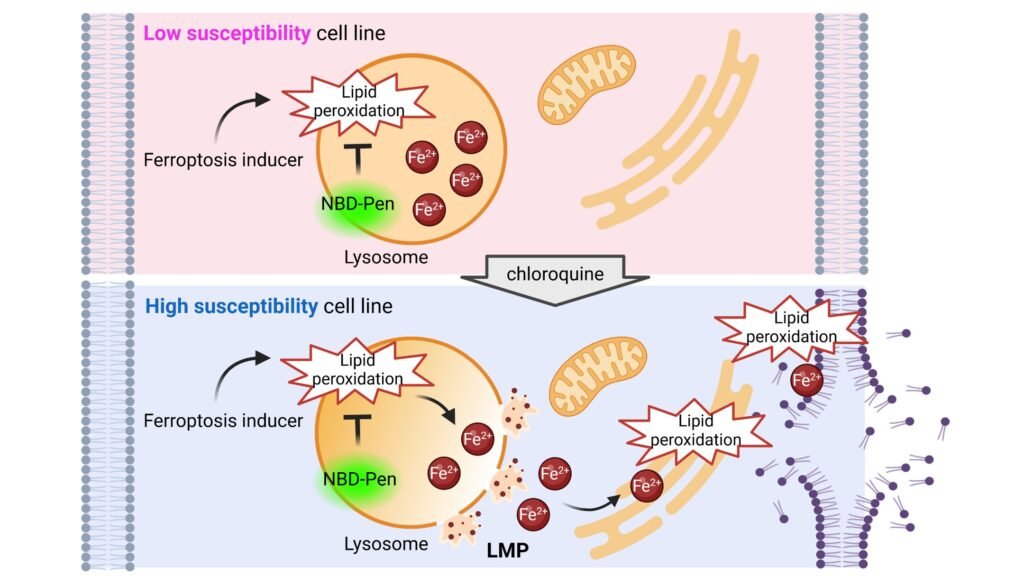
Researchers from Kyushu University recently published a study in Nature Communications, where they investigated the role of lysosomes in ferroptosis. Lysosomes are organelles responsible for breaking down and recycling molecules within cells. The team found that lipid peroxidation of lysosomes is a key factor in triggering ferroptosis. This process leads to the leakage of iron from the lysosomes, further promoting cell death. Interestingly, the researchers discovered that treating cancer cells with chloroquine, a drug that damages lysosomal membranes, can induce ferroptosis in cells that are less sensitive to this process.
Professor Ken-ichi Yamada, who led the study, explained that previous research on ferroptosis focused on the final stages of cell death, such as membrane damage. However, the origin of lipid peroxidation that initiates ferroptosis remained unclear. By visualizing lipid radicals within cells, the team identified that peroxidation of lysosomes triggers ferroptosis and causes the membrane to permeabilize, leading to iron leakage and cell death.
The researchers believe that their findings could contribute to the development of novel cancer therapies targeting ferroptosis. They also plan to explore the underlying mechanisms of ferroptosis further. While strategies to enhance ferroptosis sensitivity have been proposed, the team’s discovery of lysosomal membrane damage as a trigger for ferroptosis in resistant cells offers a new approach to overcoming this challenge.
The study, titled “Lysosomal lipid peroxidation contributes to ferroptosis induction via lysosomal membrane permeabilization,” sheds light on the intricate mechanisms of ferroptosis and opens new avenues for research in cancer treatment. By understanding how lysosomal dysfunction drives cell death, scientists can potentially develop more effective therapies for a wide range of diseases. Further investigation into the mechanisms behind ferroptosis-low-susceptibility cells is needed to fully harness the therapeutic potential of this process.