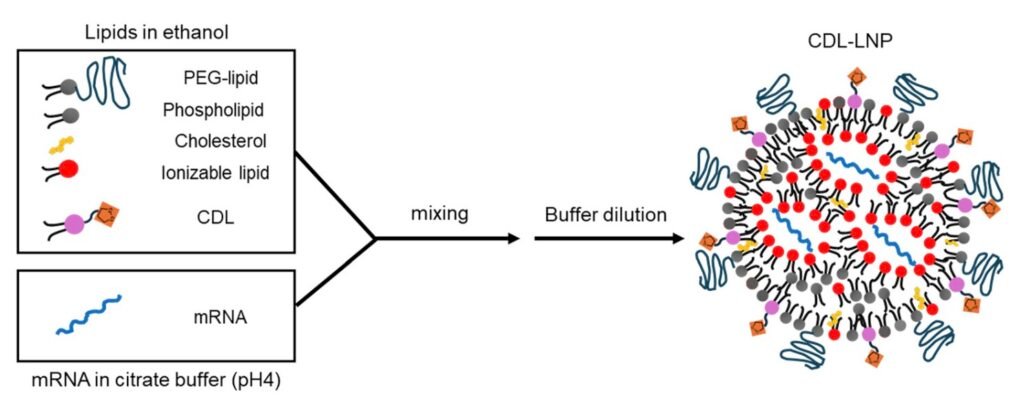
Researchers at Nagoya University in Japan have made a significant breakthrough in improving the delivery of mRNA to cells by developing a lipid nanoparticle that is five times more effective. This advancement involves attaching a cyclic disulfide—a sulfur-containing ring structure—to lipid molecules, allowing for a higher escape rate of mRNA from cellular components that typically break down genetic material. In a study published in the journal RSC Medicinal Chemistry, the researchers demonstrated the successful use of cyclic disulfide-containing lipids to enhance mRNA delivery in animals and inhibit tumor growth when tested as a cancer vaccine in mice.
The process of formulating the lipid nanoparticle involves mixing mRNA and lipids under specific conditions, leading to the formation of spherical structures that protect the mRNA inside. However, a significant challenge arises when the mRNA enters cells, as more than 95% of it becomes trapped in endosomes—small compartments within cells—that eventually degrade the genetic material. The introduction of the new disulfide-based delivery system helps the mRNA escape from these endosomes, enabling better delivery of genetic instructions to produce antigens. This, in turn, triggers the immune system to generate antibodies and activate immune cells.
Dr. Seigo Kimura, the lead author of the study from the Integrated Research Consortium on Chemical Sciences at Nagoya University, emphasized the substantial improvements in mRNA delivery observed with the modified lipids. In laboratory cell cultures and living organisms, the new formulations showed up to a 6-fold increase in efficiency in cell culture experiments and a 5-fold improvement in mice compared to standard methods. This marks the first instance of disulfide chemistry enhancing mRNA delivery in vivo and proving effective as a cancer vaccine in mice.
The researchers conducted tests using two established lipid formulations—MC3 and SM102—by enhancing them with cyclic disulfide molecules. The modified particles were initially tested on human cells in the lab to assess their mRNA delivery efficiency before being evaluated in mice to gauge their effectiveness in delivering genetic instructions and eliciting cancer vaccine responses. The results showed that the modified particles outperformed their standard counterparts, indicating the potential for these molecules to enhance existing delivery systems.
Despite the promising results, Dr. Kimura stressed that further testing in various delivery methods and safety studies is necessary before widespread implementation. With cancer being the second leading global cause of death and the challenges inherent in developing an effective vaccine, the research opens new avenues for addressing delivery efficiency, immune evasion, tumor suppression, and biological complexity in cancer treatment.
In conclusion, the development of cyclic disulfide-containing lipids for improved mRNA delivery represents a significant advancement in the field of cancer research and vaccine development. The study not only underscores the potential of disulfide chemistry in enhancing mRNA delivery but also highlights the importance of continued research to harness the full capabilities of this novel delivery system.