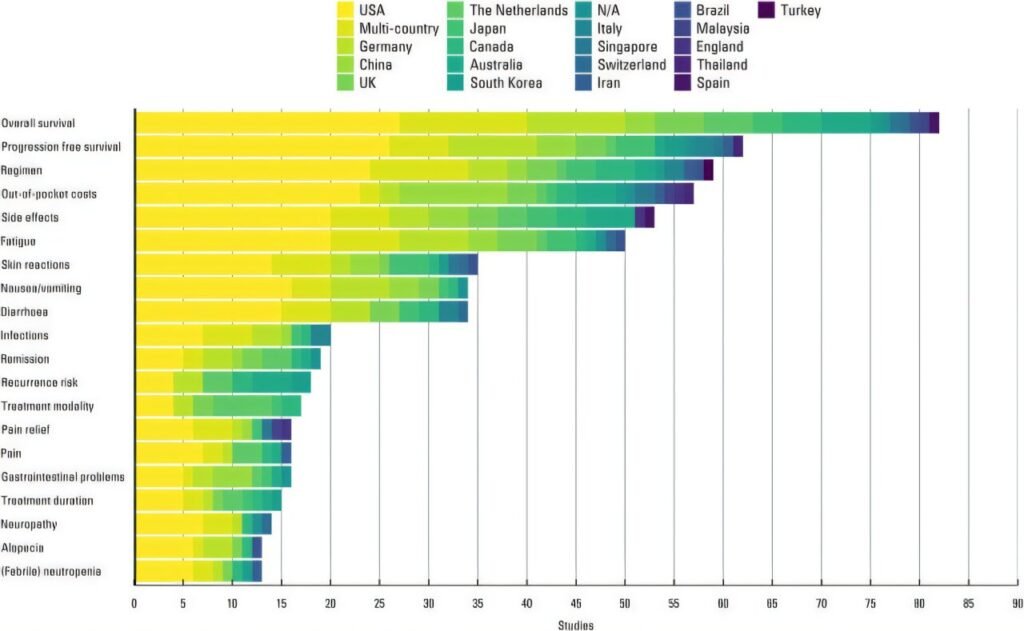
Patient preference studies play a crucial role in shaping drug development and regulatory decisions in healthcare. However, conducting these studies can be costly and time-consuming. A recent study conducted by researchers from the University of Twente and international collaborators has shed light on the potential for reusing existing data from patient preference studies to improve resource utilization in patient-focused drug development.
Published in the journal “Value in Health,” the study analyzed trends in 777 published patient preference studies. The researchers found that in certain disease areas like type 2 diabetes, there is a significant overlap in study methods and clinical focus, making it possible to combine results across studies. This opens up opportunities for “benefit transfer,” where preference evidence from multiple studies can be synthesized statistically to predict preferences in new decision-making contexts.
While some disease areas like cancer, psoriasis, and multiple sclerosis have more fragmented data, the researchers still see potential for benefit transfers. By making smarter use of existing patient preference data, decision-making in drug development can be accelerated, and past academic efforts can be sustained. This approach ensures that patient perspectives continue to influence new treatments and policies long after the original studies.
According to Michael Bui, an author from the Department of Health Technology and Services Research at the University of Twente, reusing existing data can contribute to the sustainability of research efforts and speed up decision-making processes. Benefit transfers are particularly valuable in situations where conducting new preference studies is challenging, providing an alternative way to incorporate patient perspectives in drug development.
The study also emphasizes the need for better reporting and common definitions of clinical endpoints to improve the use of existing data. Clearer guidelines could expand the application of benefit transfers to more areas of healthcare. However, the researchers stress that their findings should not discourage new preference studies, as they remain essential sources of information when existing evidence is insufficient.
For more information on this study, readers can refer to the article “Do We Always Need a New Preference Study? A Scoping Review of Promising Research Areas for Meta-Analyses and Benefit Transfers of Patient Preference Studies” published in “Value in Health” in 2025. The DOI for the article is 10.1016/j.jval.2025.06.004.
This research, conducted by the University of Twente, highlights the potential for transforming healthcare by leveraging existing patient preference data. By reusing and synthesizing data from multiple studies, the medical product lifecycle can become more sustainable, benefiting both researchers and patients in the long run.