The University of Massachusetts Amherst-Ernest Pharmaceuticals team has made significant advancements in developing a non-toxic bacterial therapy, BacID, for delivering cancer-fighting drugs directly into tumors. This innovative technology shows promise for safer and more effective treatment of high-mortality cancers such as liver, ovarian, and metastatic breast cancer.
Clinical trials involving cancer patients are expected to commence in 2027. Neil Forbes, the senior author of the research recently published in the journal Molecular Therapy, and a professor of chemical engineering at UMass Amherst, expressed excitement about the potential of this bacterial treatment for cancer. Lead author Vishnu Raman, who completed his Ph.D. in the Forbes Lab at the UMass Amherst Institute for Applied Life Sciences (IALS), highlighted the targeted nature of the treatment, which can address late-stage cancers without the harsh side effects commonly associated with traditional therapies like chemotherapy.
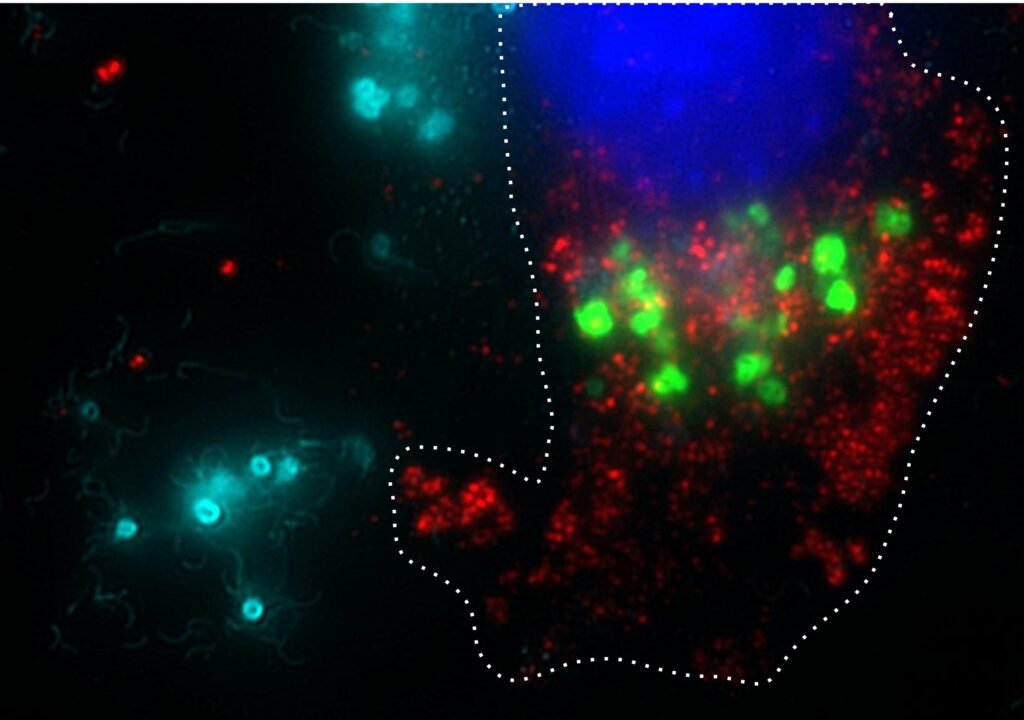
The development of genetically engineered strains of Salmonella has been a focal point of the team’s research over the past decade. These strains are designed to specifically target tumors and release cancer-fighting drugs within cancer cells, while sparing healthy tissue. This targeted approach allows for significantly higher concentrations of therapy to be delivered to tumors, as the bacteria multiply within the tumor environment.
Raman, as the chief scientific officer of Ernest Pharmaceuticals, played a key role in enhancing the safety and efficacy of the bacterial strain. By incorporating a genetic circuit that controls the invasion and therapy delivery process within cancer cells, the team has made the treatment much safer and more precise.
One of the critical components of this technology is the controlled activation of flagella, the bacterial organelles responsible for movement. The team discovered that aspirin can trigger the production of flagella within the bacteria, enabling them to invade cancer cells and deliver therapy. Additionally, a built-in suicide circuit ensures that the bacteria rupture within cancer cells, releasing the therapy effectively.
In pre-clinical studies using mouse models, the bacteria are administered intravenously and targeted to tumors. Following a brief period of exponential growth within the tumors, a simple dose of aspirin activates the invasion and therapy delivery process. This patient-friendly approach aims to streamline the treatment process, allowing patients to receive an infusion followed by an oral aspirin dose at home.
The team is now focused on obtaining regulatory approval for clinical trials, with a keen interest in advancing microbial-based cancer treatments. The potential of BacID to revolutionize cancer therapy and provide a safer, more effective alternative to existing treatments underscores the importance of ongoing research in this field.