Tooth development is a complex process that involves various stages such as bud, cap, bell, root development, and tooth formation. Epithelial-mesenchymal interactions play a crucial role in mediating processes like the bud-to-cap transition. Additionally, the positional identity of cells within the developing embryo determines their fate, influenced by the concentration of signaling molecules and growth factors.
Researchers, led by Dr. Han-Sung Jung from Yonsei University College of Dentistry, Korea, conducted a study to investigate how the position of young epithelial and mesenchymal dental cells impacts their development. Their findings, published in the International Journal of Oral Science, shed light on the mechanisms governing tooth development.
The team isolated mesenchymal cells from the lingual and buccal sides at the cap and bell stages of mouse embryo development. By comparing their gene expression profiles through RNA-seq and gene ontology enrichment analysis, they identified distinct differences based on position and time. Subsequent transplantation of these cells under the kidney capsule of immunocompromised mice revealed that lingual cells primarily contributed to tooth formation, while buccal cells were involved in stem cell activity and supporting tissue growth.
Further experiments involved mixing tagged lingual and buccal cells from genetically engineered mice to observe their self-organizing capabilities. The results demonstrated that the cells could reorganize and maintain their original positions, with lingual cells forming tooth enamel even when mixed randomly. This phenomenon, known as cellular self-organization, highlights the remarkable plasticity of dental mesenchymal cells.
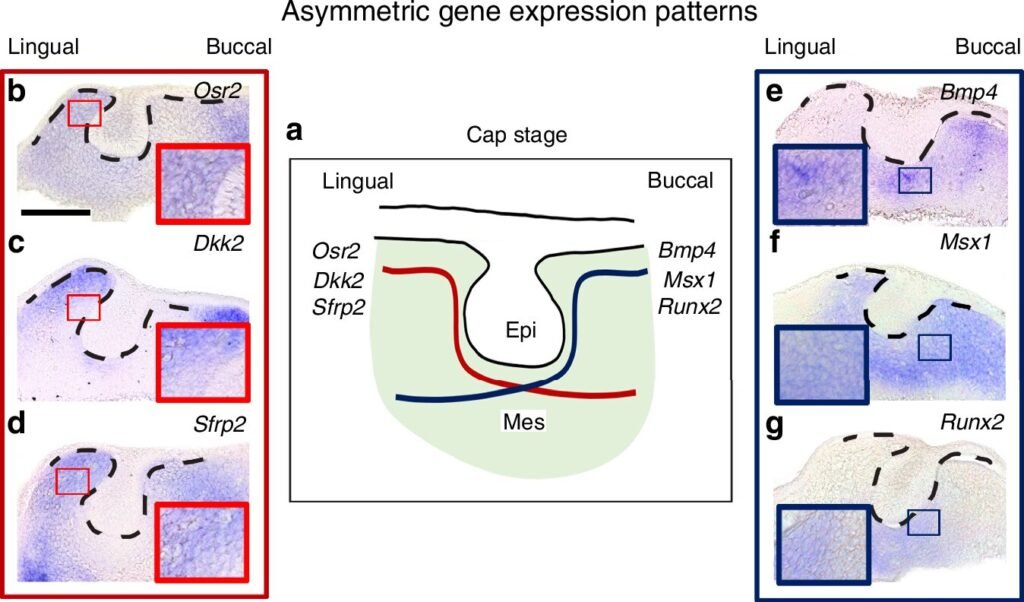
Analysis of signaling molecules revealed that lingual cells exhibited enriched WNT signaling and R-spondins, promoting tooth formation through increased proliferation and migration. In contrast, buccal cells showed higher expression of BMP inhibitors, lower proliferation, and slower migration, favoring the formation of bone and surrounding tissues.
Based on their findings, the researchers proposed a model of dental cell positioning along the lingual-buccal axis, where the fate of tooth and tissue formation is determined by the differential expression of WNT/BMP signaling molecules. This deep understanding of the molecular mechanisms underlying tooth development holds promise for advancements in tissue engineering, regenerative medicine, and stem cell-based tooth regeneration.
Ultimately, this research paves the way for more effective therapeutic strategies in dental restoration and repair, harnessing the intricate interplay of dental mesenchymal cells to drive innovative treatments in the field of dentistry.