UCLA researchers have made a groundbreaking discovery regarding how cells, particularly cancer cells, survive and thrive in low-nutrient environments by activating a growth protein called MYC and altering chromatin structure. This finding sheds light on how cells adapt and continue to grow even when faced with metabolic stress due to nutrient scarcity.
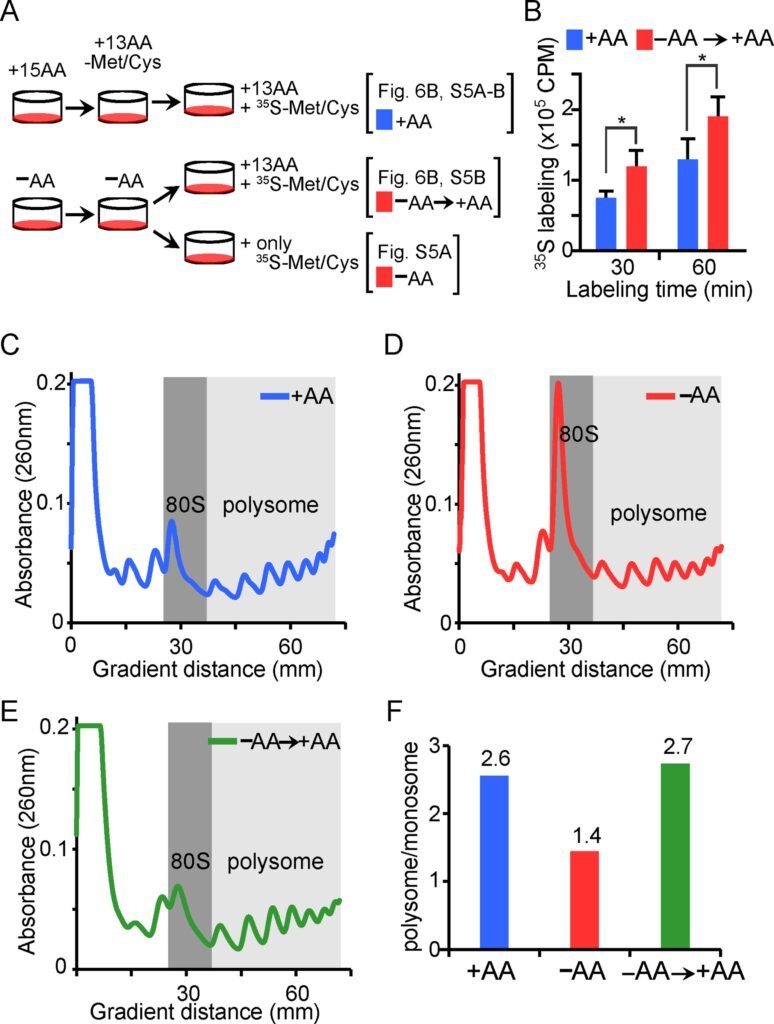
When cells are deprived of amino acids, essential for protein synthesis and growth, they trigger the activation of the growth protein MYC and reduce a specific chemical marker known as H4K20me1 on active genes. These simultaneous changes prepare the cell to rapidly increase protein production once nutrients become available again. This unexpected boost in protein synthesis capacity during nutrient deprivation could explain how cancers driven by MYC manage to survive and proliferate despite limited resources.
The MYC gene, commonly overactive in various cancers, plays a crucial role in enhancing protein production. However, the epigenetic mechanisms that collaborate with MYC to support this process, especially during amino acid deficiencies, have been unclear until now. By studying human cells grown with and without amino acids, researchers observed how DNA packaging and gene activity change in response to nutrient shortages using molecular tools to measure gene expression and protein production.
These findings pave the way for novel approaches in targeting cancer metabolism and growth by uncovering a chromatin-based strategy that primes cells for protein synthesis even in nutrient-poor conditions. Understanding how MYC aids cells in surviving and recovering from nutrient stress may lead to innovative treatment strategies for MYC-driven cancers and other diseases associated with metabolic challenges.
Dr. Siavash Kurdistani, the senior author of the study and a professor at the David Geffen School of Medicine at UCLA, highlighted the significance of this priming mechanism in enabling cells to adapt and survive under stress. This newfound knowledge could offer insights into disrupting cancer cells’ ability to thrive under adverse conditions.
The study, titled “Coordinated histone methylation loss and MYC activation promote translational capacity under amino acid restriction,” was published in Cancer & Metabolism. Understanding how cells navigate nutrient scarcity to prepare for a surge in protein synthesis may revolutionize cancer treatment strategies and shed light on the mechanisms underlying cellular adaptation to stress.
For more information, you can access the full study published in Cancer & Metabolism by Chen Cheng et al. with DOI: 10.1186/s40170-025-00399-x. This research was conducted at the University of California, Los Angeles, and offers valuable insights into cellular survival strategies in challenging environments.