Snake antivenom serum has been a crucial lifesaving treatment for snakebite envenomation for over a century. In Brazil, a groundbreaking study conducted by researchers from the Federal University of São Paulo (UNIFESP) and the Butantan Institute has led to the development of a new and improved version of the antibothropic serum. This new serum has proven to be three times more effective than the standard serum currently used to treat snakebites from the Bothrops genus, particularly the jararaca snake (B. jararaca).
Published in the Journal of Proteome Research, the study led by Professor Alexandre Tashima focused on enhancing the potency and safety profile of the antivenom serum. By combining traditional methods with cutting-edge techniques, the researchers were able to increase the concentration of proteins that neutralize venom while reducing potentially harmful molecules that could cause side effects.
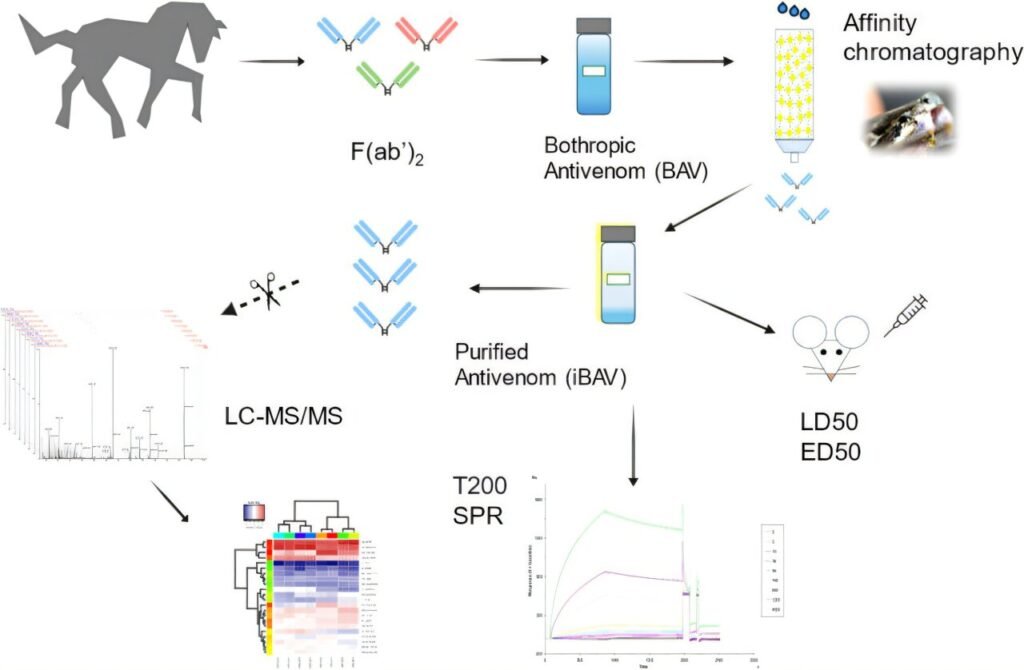
The process of producing antivenom serum involves injecting non-lethal doses of snake venom into large animals, such as horses, to stimulate the production of antibodies against the toxins. After harvesting the antibody-enriched blood from the animals, it undergoes purification to create the heterologous serum, the gold standard treatment for snakebite envenomation.
However, the standard antibothropic serum was found to contain only 27.8% of proteins that effectively interacted with the toxins in the jararaca venom. The remaining components included non-specific antibodies and horse albumin, which can trigger adverse immune responses in patients receiving the serum.
To address these limitations, the researchers developed a new purification process using affinity chromatography to isolate the antibodies that bind specifically to the venom toxins. This improved serum showed a significant reduction in horse albumin by 87% and other proteins by up to 83%, resulting in a serum with 2.9 times greater affinity for venom toxins.
Animal studies demonstrated that mice treated with the new serum required 2.8 times less dosage to counteract the venom’s effects, indicating increased potency and reduced risk of adverse reactions. While the technologies for this purification stage are readily available, further clinical and regulatory research is needed before the improved serum can be introduced as a new treatment option.
Looking ahead, advancements in technology, such as monoclonal antibodies, hold promise for the development of more targeted and efficient treatments for snake envenomation. Despite these innovations, the heterologous serum is likely to remain a critical treatment for snakebite envenomation, considering the high global burden of snakebite cases, particularly in rural and underserved communities.
The World Health Organization (WHO) has recognized snakebite poisoning as a neglected tropical disease, highlighting the importance of continued support for research and initiatives aimed at reducing the morbidity and mortality associated with snake envenomation. The innovative research conducted by the Brazilian team represents a significant step towards improving the efficacy and safety of antivenom treatments, ultimately saving more lives from the devastating effects of snakebites.