As we age, our bodies undergo various changes, and this includes our bones. A recent study led by the University of Texas at Austin, in collaboration with Mayo Clinic and Cedars-Sinai Medical Center, has shed light on the aging process of osteocytes, cells that play a crucial role in maintaining bone strength. The research, published in Small and Aging Cell, reveals how aging and stress can induce cellular senescence in osteocytes, leading to structural and mechanical changes that weaken bones over time.
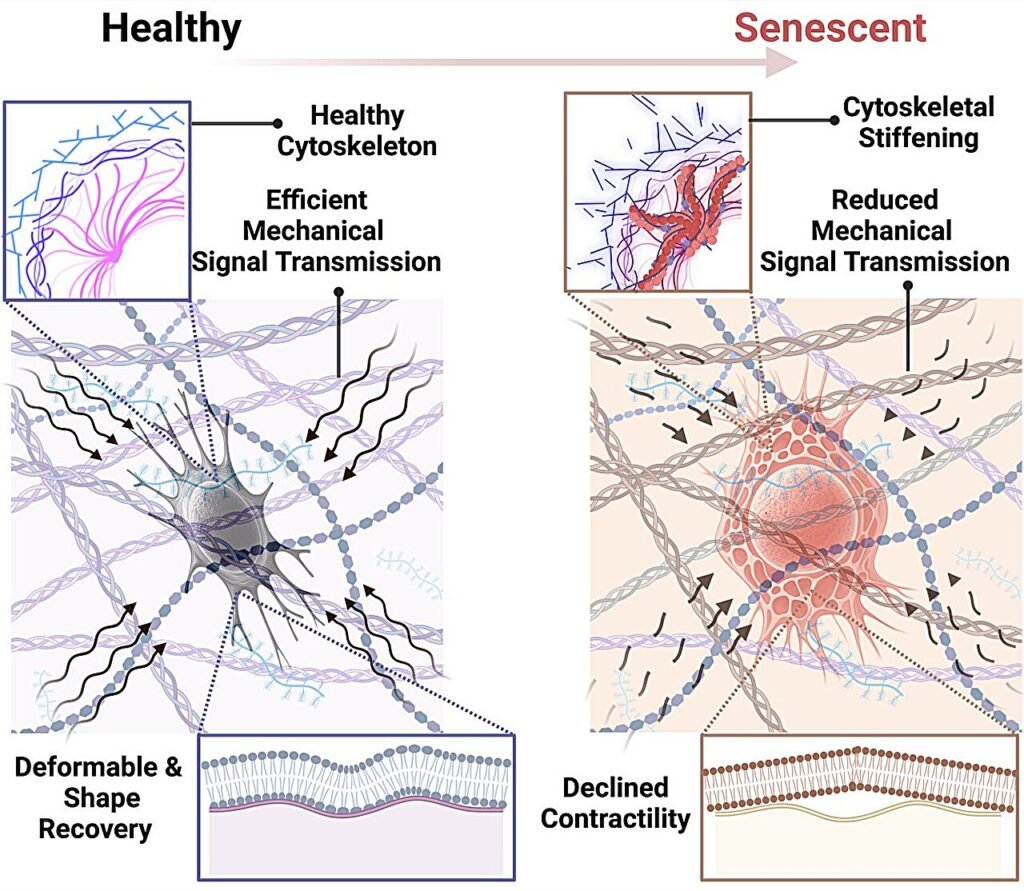
Osteocytes are responsible for regulating bone health by sensing mechanical forces and coordinating bone remodeling. However, when these cells are exposed to senescent cells, which are damaged cells that stop dividing, they begin to stiffen. This stiffness, along with altered plasma membrane viscoelasticity, impairs their ability to respond to mechanical signals, disrupting the normal bone remodeling process and resulting in bone fragility.
Maryam Tilton, an assistant professor at the Cockrell School of Engineering, compared the cytoskeleton of osteocytes to the scaffolding inside a building. When this scaffolding becomes rigid, the building becomes less adaptable to changes and stresses, leading to structural issues. Similarly, stiffened osteocytes cannot effectively regulate bone remodeling, contributing to bone loss.
Senescent cells release a mix of molecules called senescence-associated secretory phenotype (SASP), which can trigger inflammation and damage in surrounding tissues. While previous research has focused on genetic markers to detect senescence, Tilton and her team took a different approach by focusing on the mechanical aspects of the cells. By combining genetic and mechanical approaches, they hope to develop improved treatments for aging cells.
Dr. James Kirkland, a principal investigator of the National Institutes of Health Translational Geroscience Network, emphasized the potential of biomechanical markers in identifying and targeting senescent cells. These markers could serve as precise targets for eliminating aging cells, offering alternatives to current drug-based therapies.
Understanding how bones age is crucial for improving treatments for conditions like osteoporosis, which affects millions of people globally, especially those over 50. The team plans to further explore the effects of different stressors on osteocytes and investigate potential therapeutic interventions to combat age-related bone loss.
This groundbreaking research provides valuable insights into the cellular mechanisms behind bone aging and offers hope for more effective treatments in the future. By combining genetic and mechanical approaches, researchers aim to develop innovative therapies to preserve bone health and quality of life as we age.