Regulatory T cells (Treg cells) are a crucial component of the human immune system, playing a vital role in suppressing harmful immune responses and promoting tissue regeneration. These specialized cells release healing substances and support regenerative cells like tissue stem cells, working in tandem with both immune and non-immune cells to facilitate the healing process. Given their diverse functions, Treg cells are being explored as potential candidates for therapeutic applications to enhance tissue function post-inflammation or in autoimmune conditions.
To support wound healing, Treg cells undergo a transformation into tissue-Treg cells, a process that remains poorly understood. Researchers from the Division of Immunology at the LIT, in collaboration with experts from the University Medical Center Mainz and the German Cancer Research Center, have delved into this differentiation process, focusing on epigenetic changes in DNA. Their study, published in Nature Immunology, sheds light on the molecular mechanisms driving the development of tissue-Treg cells in humans.
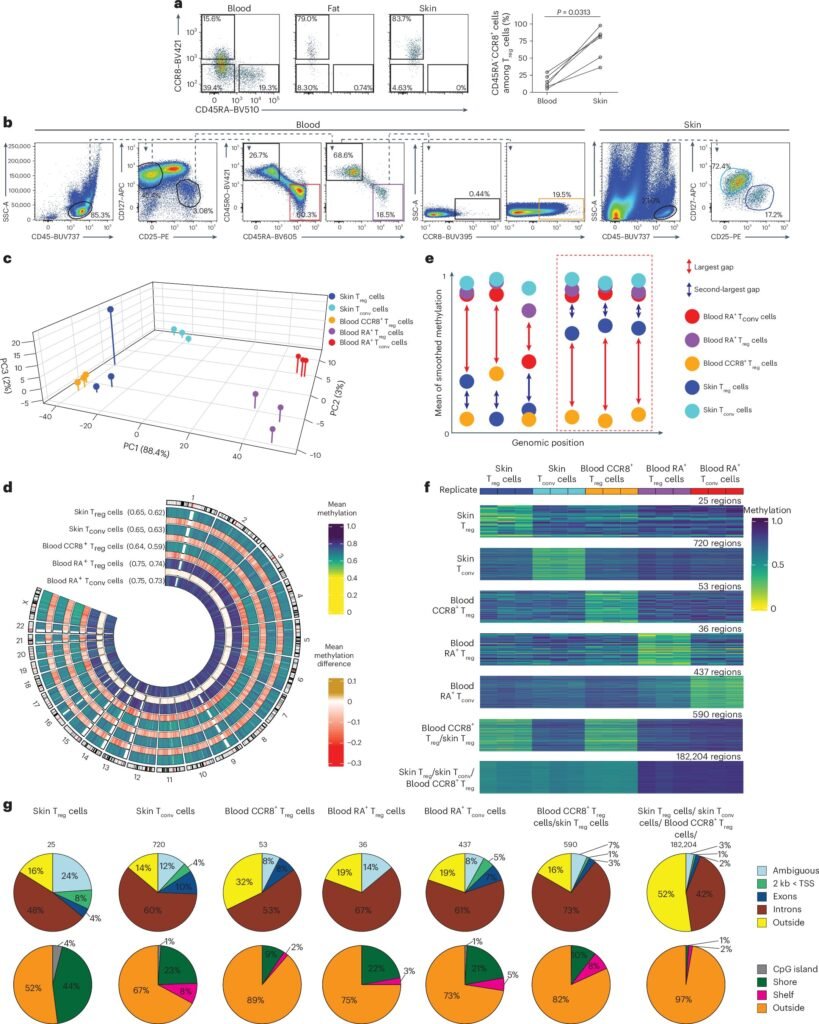
Epigenetics, the study of changes in gene function that do not involve alterations in the DNA sequence, plays a crucial role in regulating gene expression. DNA methylation, the addition of a methyl group to specific sites on DNA, influences gene activation by acting as an ‘on’ or ‘off’ switch. By analyzing DNA methylation patterns in Treg cells, the researchers identified key regulatory elements that govern the transition to tissue-Treg cells.
The DNA methylation profile of tissue-Treg cells serves as a unique identifier, distinguishing them from other cell types. This ‘genetic fingerprint’ not only helps in characterizing tissue-Treg cells but also enables the identification of circulating tissue-Treg cells in the bloodstream. Furthermore, the study revealed significant changes in transposable elements, or ‘jumping genes,’ highlighting their role in gene regulation and cell function.
These findings open up new avenues for leveraging tissue-Treg cells in therapeutic interventions for various diseases. From enhancing tissue function post-inflammation to treating autoimmune disorders, the insights gained from this research offer promising prospects for harnessing the regenerative potential of Treg cells. By unraveling the intricate mechanisms underlying Treg cell differentiation, this study paves the way for innovative treatment strategies aimed at leveraging the body’s natural healing processes for improved clinical outcomes.