A groundbreaking study led by Stephen D. Nimer, M.D., director of Sylvester Comprehensive Cancer Center at the University of Miami Miller School of Medicine, has shed light on the regulation of blood cell generation through a key molecule known as TAF1. This molecule plays a crucial role in hematopoiesis, the process by which new blood cells are formed, a process that is often disrupted in cancer.
Published in the prestigious journal Developmental Cell, the study has unveiled potential therapeutic strategies that target TAF1 as a regulator of gene activity. According to Sylvester researcher Ramin Shiekhattar, Ph.D., who is also the co-leader of the Cancer Epigenetics Program at Sylvester, the findings not only challenge existing models of hematopoietic regulation but also pave the way for innovative clinical applications.
Previous research by Nimer, Shiekhattar, and their team demonstrated that TAF1 suppression effectively suppressed disease progression in a model of acute myeloid leukemia driven by the oncogenic AML1-ETO fusion protein. It was revealed that TAF1 collaborates with AML1-ETO to activate genes implicated in cancer development.
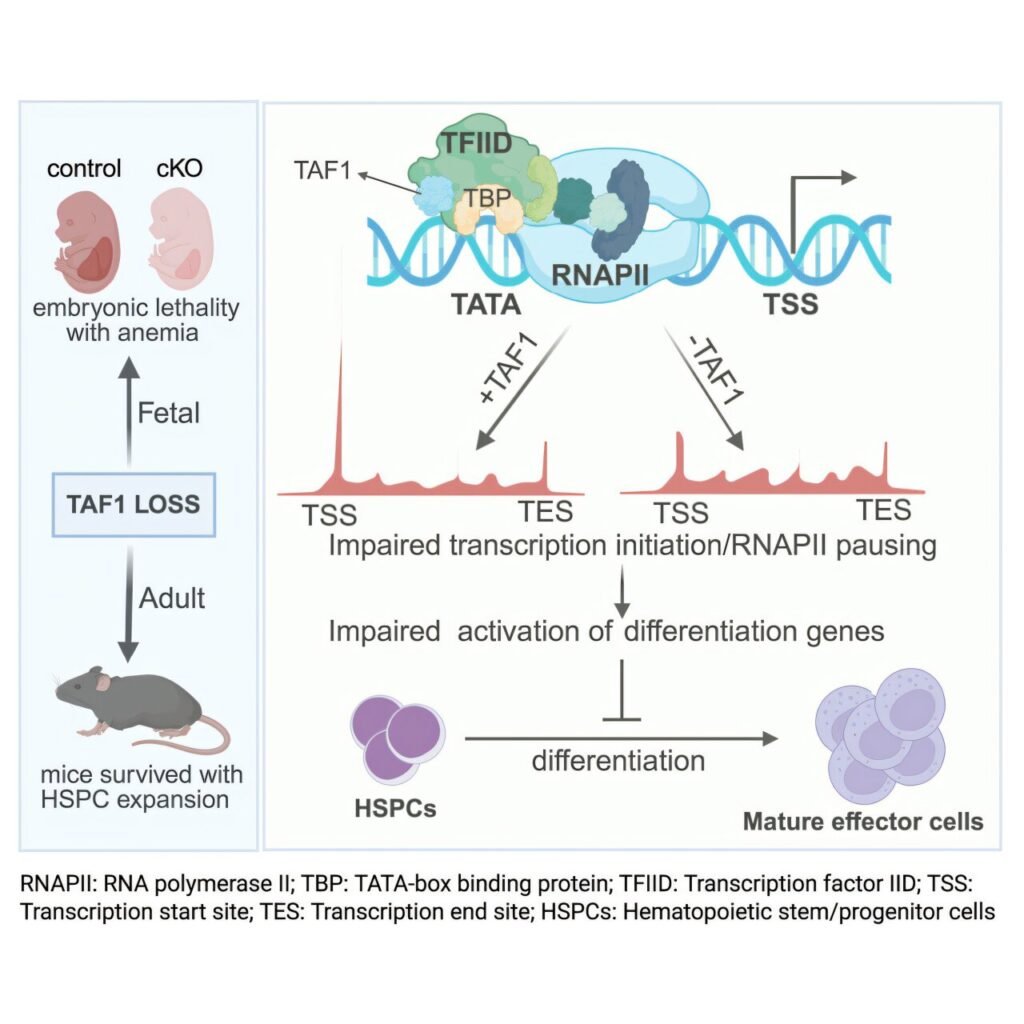
TAF1 is a component of a large molecular complex that binds to DNA and facilitates gene activation. In the current study, researchers delved deeper into the role of TAF1 in normal blood cell development, particularly in the differentiation of hematopoietic stem cells (HSCs) into various mature blood cell types.
HSCs are pivotal cells found in the bone marrow that possess the unique ability to self-renew and give rise to different blood cell lineages. The study indicated that TAF1 is crucial for activating genes involved in lineage commitment in adult blood cell development, while its role in HSC self-renewal is relatively diminished. Interestingly, TAF1 operates differently during embryonic blood cell development, suggesting a more pronounced role in blood production during early developmental stages.
The research team’s findings challenge the conventional understanding of TAF1’s function, highlighting its specialized role in regulating genes that drive HSC differentiation into mature blood cells. Moreover, the study identified TAF1 as a key molecular switch that coordinates transcriptional signals to balance stem cell maintenance with lineage commitment in adults.
Furthermore, the study uncovered the dual role of TAF1 in initiating transcription and releasing a brake on the transcription process, shedding light on its intricate mechanism of action. Future investigations will explore whether TAF1 plays similar roles in other stem cell populations relevant to cancer.
The potential therapeutic implications of targeting TAF1 are promising, with ongoing efforts to develop TAF1-targeting agents. These agents could offer a targeted approach to cancer treatment by selectively inhibiting cancer cell growth while preserving normal blood cell development. Additionally, TAF1 inhibitors may enhance the expansion of HSCs in laboratory settings, potentially improving stem cell transplantation outcomes.
The study’s findings provide a solid foundation for future research endeavors and offer hope for the development of novel therapeutic strategies in cancer treatment, particularly in the realm of blood cell disorders. The intricate role of TAF1 in blood cell development underscores its significance as a potential therapeutic target and opens up new avenues for precision medicine approaches in oncology.
As the scientific community continues to unravel the complexities of TAF1’s function, the study’s implications hold promise for advancing our understanding of blood cell regulation and paving the way for innovative therapeutic interventions in cancer treatment.