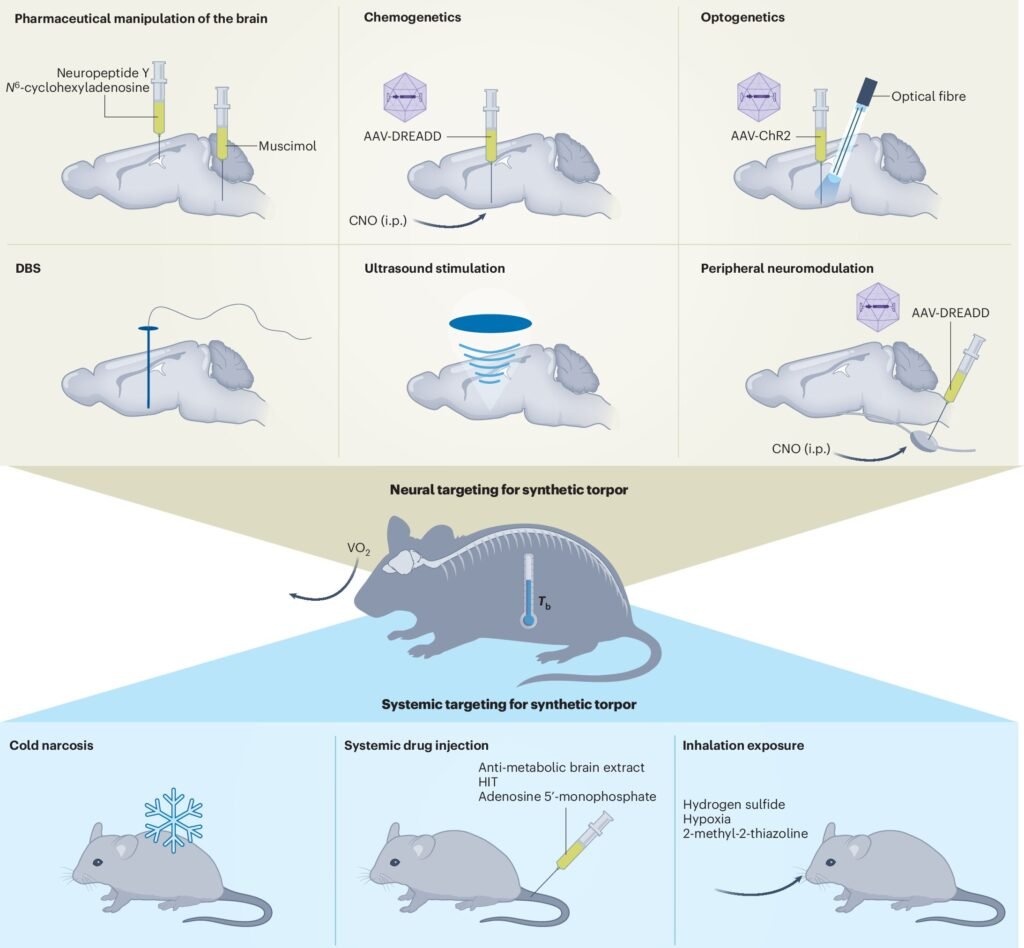
Nature has always been a source of inspiration for scientific breakthroughs, and the concept of torpor is no exception. Torpor is a state in which some mammals and birds can lower their body temperature and metabolic rate to survive extreme conditions. Researchers have long been intrigued by this phenomenon, seeking to replicate it in humans for various medical applications.
A recent study led by Hong Chen, a professor of biomedical engineering and neurosurgery at Washington University in St. Louis, has made significant strides in inducing a torpor-like state in mice using focused ultrasound. By targeting the hypothalamus preoptic area in the brain, which regulates body temperature and metabolism, the team was able to induce reversible torpor in mice and even in rats, which do not naturally enter torpor.
The research, published in a Perspectives paper in Nature Metabolism, showcased the first noninvasive and safe method to induce torpor-like states by targeting the central nervous system. This approach holds promise for a wide range of medical applications, such as preserving organs for transplantation, protecting against radiation during space travel, and reducing energy demand in various medical interventions.
While the concept of synthetic torpor is groundbreaking, there are still challenges to overcome before it can be applied in humans. Issues such as metabolic differences between animals and humans, determining the right dosage of medication, and ensuring reversibility of the torpor-like state need to be addressed. Collaboration among scientists, clinicians, and ethicists will be crucial in developing safe and effective solutions for synthetic torpor in medicine.
The team also developed a wearable ultrasound transducer to stimulate neurons in the hypothalamus preoptic area, resulting in a drop in body temperature and metabolic changes characteristic of torpor in mice. This noninvasive approach using ultrasound shows promise for inducing synthetic torpor without the need for genetic modifications.
In addition to its potential medical applications, synthetic torpor could also be used to inhibit tumor growth and develop new therapies for neurodegenerative diseases like Alzheimer’s. However, more research is needed to understand how different brain regions, peripheral organs, and cellular pathways coordinate metabolic suppression and arousal.
The future of synthetic torpor holds great promise for revolutionizing medicine, but it will require interdisciplinary collaborations, further preclinical studies, and technological innovations to translate this concept from theory to practical applications. By bridging fundamental neuroscience, bioengineering, and translational medicine, synthetic torpor could become a reality in the near future, offering new possibilities for medical interventions and treatments.
As we continue to unravel the mysteries of torpor and its potential applications in medicine, the possibilities are endless. Synthetic torpor could redefine the way we approach various medical conditions and interventions, opening up new avenues for research and treatment. The journey towards harnessing the power of torpor in medicine is just beginning, and the future looks bright with possibilities.