A recent global study conducted by researchers at the School of Clinical Sciences at Monash Health, Monash University, has shed light on the importance of transparent data-sharing in clinical trials. The study, published in eClinicalMedicine, focused on 265 randomized controlled trials involving over 65,000 women and examined the impact of data-sharing on the trustworthiness and quality of the trials.
Lead author Dr. Malitha Patabendige, an Obstetrics and Gynecology Registrar at Monash Health and Ph.D. candidate at the School of Clinical Sciences, emphasized that the goal of the study was not to determine the benefits of labor induction but rather to assess the reliability of the research and the impact of transparency on patient care. The researchers used the TRACT checklist to evaluate each study across several key areas, including ethical approvals, reporting quality, and consistency of results.
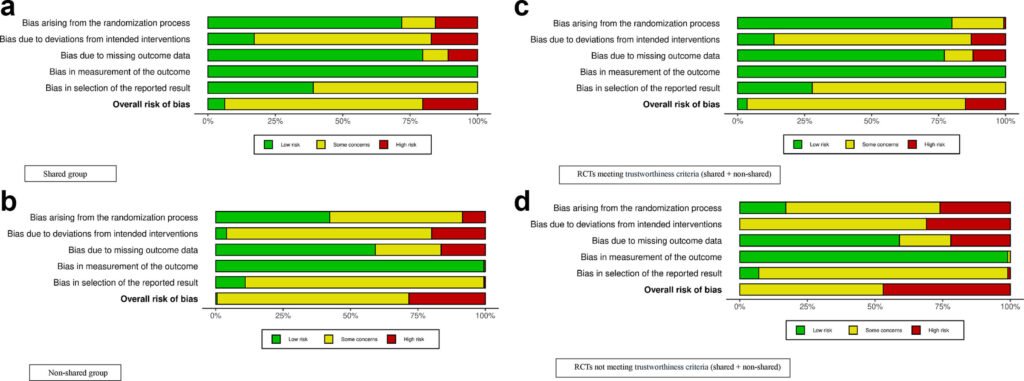
The study revealed that trials which shared their original datasets were more likely to be well-designed, transparent, and reliable compared to those that did not share data. Only 24% of the trials included in the analysis shared their original data, with 84% of those meeting trustworthiness criteria. In contrast, more than half of the trials that did not share data raised concerns about inconsistencies, missing information, or study design flaws.
Dr. Patabendige highlighted the importance of data-sharing as a filter for evidence, enabling the identification of strong and transparent trials. Given that labor induction is a common intervention in maternity care, with more than one in three births in Australia involving induction, the quality of the underlying evidence is crucial. The authors stressed that while data sharing does not guarantee quality, it allows for greater scrutiny and signals scientific integrity.
The researchers called for increased support and expectations for sharing de-identified trial data, particularly in fields like pregnancy and women’s health where evidence directly impacts clinical care. They emphasized that sharing data strengthens the scientific process, rebuilds trust, and ultimately leads to better decision-making for patients.
For more information on the study, readers can refer to the publication in eClinicalMedicine by Malitha Patabendige et al., titled “Data-sharing and trustworthiness of trials evaluating cervical ripening in induction of labour.” The DOI for the study is 10.1016/j.eclinm.2025.103346.
This study underscores the importance of transparency in clinical research and the need for greater accountability in the sharing of trial data to ensure the trustworthiness and reliability of medical evidence.